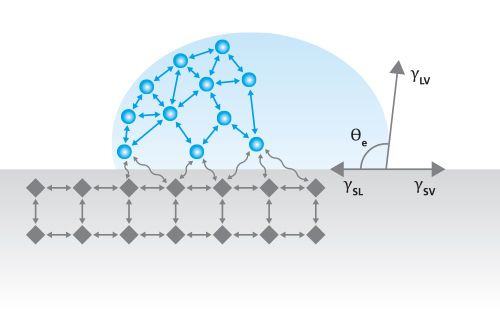
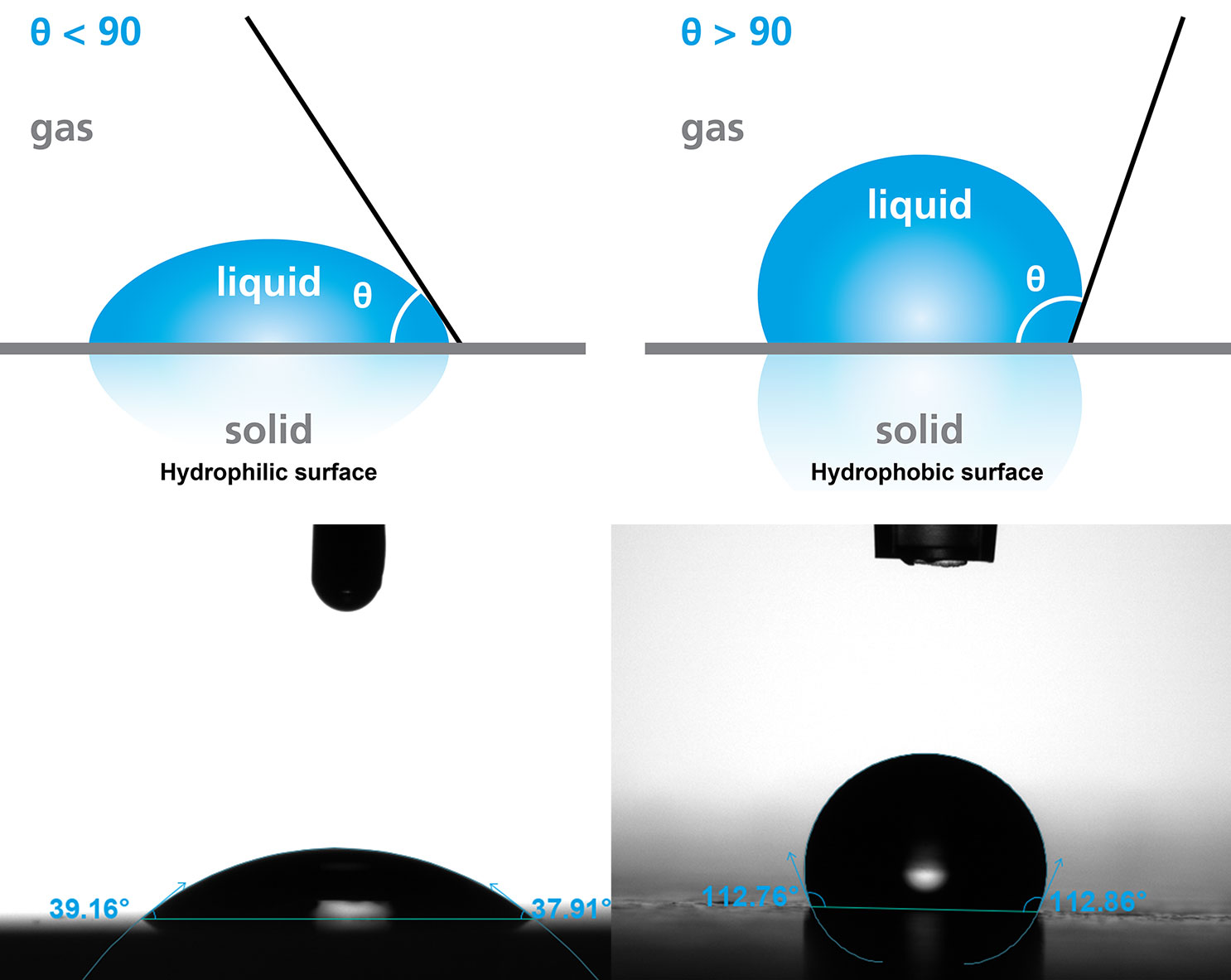
Contact angle is defined as the angle formed by a liquid at the three-phase boundary where the liquid, solid, and gas intersect. It gives a fast, quantitative measure of a surface’s wetting properties. These properties can give insight into a solid surface’s interaction with liquids, such as its adhesion with a coating or its biocompatibility with bodily fluids. Contact angle measurements are used in a wide variety of applications, including evaluating surface cleanliness, characterizing functional coatings, and assessing surface treatments.
In theory, any liquid (probe liquid) can be used for measuring contact angles. However, in most cases, certain liquids are preferred either due to convenience or necessity. Certain measurements require choosing the right probe liquid. This article briefly discusses the use of the ideal probe liquid for each measurement.
Measuring Wettability:
Wettability refers to the tendency of a liquid to spread across a surface of interest. The wetting behavior of a liquid on a solid can be quantified with contact angle. Contact angles below 90° indicate good wetting, while angles above 90° indicate poor wetting. Water is the most common probe liquid for wettability studies due to its ubiquity, low cost, and non-toxicity. Surfaces with good wetting by water are classified as hydrophilic, while surfaces with poor wetting by water are classified as hydrophobic.

While water is the most used liquid for wettability measurements, virtually any liquid can be used. In many applications, the wettability of non-water liquids is of interest. For example, the wettability of a paint with a surface can affect the paint’s performance, or the wettability of a lead-free solder can affect its solderability. In cases like these, using the liquid of interest directly as a probe liquid can give a better picture of wettability than using water. A paint’s wettability with a surface can be quantified using the paint as the probe liquid, or solder’s wettability can be quantified using the solder as a probe liquid. In both these cases, a low contact angle indicates greater wettability.
Characterizing Oleophobicity:
Modern materials like smudge-resistant smartphone screens or anti-fog glasses use oleophobic surfaces. Oleophobic surfaces are oil-repellant or, more specifically, resistant to the adhesion or penetration of oils. Typically, oleophobic surfaces are hydrophobic and can be somewhat characterized by water contact angle. However, not all hydrophobic surfaces are oleophobic and not all oleophobic surfaces are hydrophobic, so water contact angle alone cannot give the best measure of oleophobicity. Oleophobic surfaces are best characterized by the contact angle of a short-chain alkane with the surface. Short-chain alkanes have low surface tension and are highly nonpolar, like oil, and, therefore, give a better measure of the surface’s interaction with oily substances. While many alkanes have been used to measure oleophobicity with contact angle, n-hexadecane is considered the standard probe liquid. Hexadecane contact angles of about 60°-80° indicate an oleophobic surface.
Determining Surface Free Energy:
Surface free energy (SFE) is a quantitative measure of the intermolecular forces at the surface. It dictates how a solid behaves when in contact with liquid and can predict adhesion between the two phases. While water contact angle can give an estimate of SFE, SFE is independent of the liquid used and cannot be measured directly. SFE is instead determined indirectly using a mathematical model and contact angle measurements with different probe liquids.
One of the most common SFE theories is the Extended Fowkes or OWRK method. This method expresses the SFE in terms of a dispersive component and a polar component and is given by the equation:
√(γLVp γSVp ) + √(γLVd γSVd ) = 0.5γLV(1+cosθ)
where γsvp is the solid’s polar SFE component, γsvd is the solid’s dispersive SFE component, γslp is the liquid’s polar surface tension component, γsld is the liquid’s dispersive surface tension component, γsl is the liquid’s total surface tension, and θ is the measured contact angle of the liquid on the solid surface. Because there are two unknowns in this equation (γsvp and γsvd), the contact angle must be measured with a minimum of two probe liquids. However, not just any liquid can be used as a probe liquid. To solve the equation, the liquids must have known surface tension components (which can be determined experimentally or from literature), and one of the liquids must be purely dispersive.
There are many purely dispersive liquids; however, most are not suitable for SFE measurements. Most purely dispersive liquids have low surface tension, which will result in the complete wetting of most solids. To have a measurable contact angle on most solids, more exotic solvents are needed. Di-iodomethane and α-bromonaphthalene are the most used dispersive liquids for SFE measurements. Both liquids have high enough surface tension to produce a measurable contact angle on most materials.
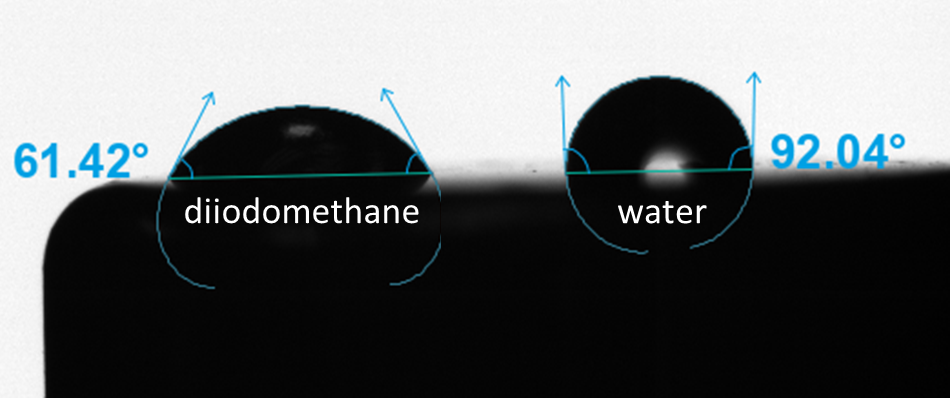
In addition to a dispersive probe liquid, a polar probe liquid is also needed for the SFE measurement. Commonly used polar probe liquids are water, ethylene glycol, glycerol, and formamide. These liquids contain heteroatoms like nitrogen and oxygen that make them capable of polar interactions with the surface.
Predicting Adhesion:
Adhesion is the attraction between two dissimilar phases and is important to all applications where two different materials are bonded together. For example, adhesion affects the application of a coat of varnish or bonding with the help of an adhesive. Good adhesion requires good wettability, so contact angle measurements can be used to evaluate a surface before bonding. Contact angle measurement between an adhesive or a coating formulation can be used to determine the optimal surface treatment or coating formulation for a particular surface. Low contact angle values indicate good wettability, and, therefore, good adhesion.
The adhesion of different coating formulations for a given surface can be evaluated using contact angle measurements. In some cases, the water contact angle is sufficient for evaluating adhesion, particularly if the coating formulation is water-based. A low water contact angle will indicate good wettability and thus adhesion with the coating formulation. However, in other cases, the coating formulation itself may make a better probe liquid for the contact angle measurement. For example, water may not give a complete picture of a surface’s wettability with an oil-based coating, particularly if the surface is hydrophobic since water will have poor wettability. In this case, the contact angle of the coating formulation on the surface can be used to evaluate wettability and adhesion. Using the coating formulation as a probe liquid also allows for comparison between different formulations, with the formulation with the lowest contact angle on the surface having the best wettability and adhesion.