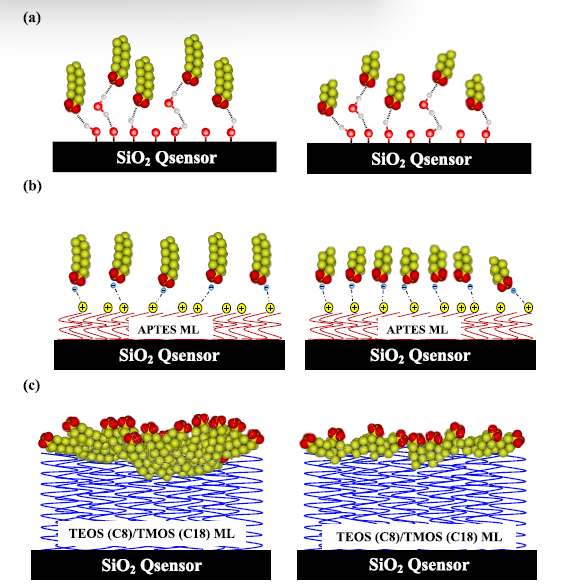
In the KORES (Karanikola Optimized Research for Environmental Sustainability) lab led by Dr. Vicky Karanikola, at the Department of Chemical & Environmental Engineering at the University of Arizona, graduate student McKenna Dunmyer and her colleagues are advancing research on per- and polyfluoroalkyl substances (PFAS) by leveraging Quartz Crystal Microbalance with Dissipation (QCM-D) technology. This highly-sensitive technique enables real-time observation of PFAS adsorption behavior on engineered sorbent materials, offering valuable insights into the mechanisms involved. The QCM-D’s unique data has been instrumental in revealing the kinetics of PFAS interactions with organosilane-functionalized silicon surfaces. The team’s research highlights the roles of electrostatic and hydrophobic forces in PFAS adsorption, providing a foundation for enhanced remediation methods to remove these persistent contaminants from water sources.

Why is PFAS Research so Critical?
PFAS (per- and polyfluoroalkyl substances) have been used in consumer products since the 1940s because of their useful properties, such as stain- and water-resistance, and fire-retardance. These manufactured “forever chemicals” are notoriously stable and resist natural degradation processes, resulting in their persistence in the environment (soil, ground water, oceans, etc.) and accumulation in the blood and tissues of humans and animals.1
One of the main thrusts of the environmental engineering community at large, and specifically Dr. Karanikola’s research, is finding a way to remove these PFAS molecules from the environment. The extremely low concentrations of PFAS recorded in environmental samples (on the order of parts per billion or even parts per trillion) make studying their environmental behavior/interactions a non-trivial effort. There are very few analytical tools with the sensitivity necessary to measure in this regime.
“Chemicals behave differently at extremely low and high concentrations, so it is important to study them at these real-world concentrations.”
Liquid Chromatography-Mass Spectrometry (LC-MS) is the most widely used technique for detecting and quantifying PFAS.2 However, LC-MS is costly, and cannot monitor changes occurring in the sample in real-time. Dr. Karanikola states, “With QCM-D, we can avoid using the expensive, time-consuming LC-MS instrument, and get real-time results on the molecular scale.”
“We started with a very applicable idea for a PFAS treatment method, but quickly realized that we needed to go back to the fundamentals. We realized that there is not enough information about how PFAS will behave with novel sorbents. That is how we got into using QCM-D to study PFAS.”
QSense QCM-D
Quartz Crystal Microbalance with Dissipation (QCM-D) is an advanced analytical technique used to study surface interactions and material properties at the nanoscale. It works by measuring changes in the resonance frequency of a quartz crystal when mass, such as molecules or particles, adsorbs onto its surface. Additionally, QCM-D captures dissipation, which provides information about the viscoelastic properties and structural changes of the adsorbed layer. This dual measurement capability makes it a powerful tool for real-time monitoring of interactions, such as adsorption processes, with high sensitivity.
The QSense QCM-D systems are the most sensitive instruments available for quartz crystal microbalance with dissipation measurements. These systems feature sensors that can be fabricated from over 100 different materials, allowing researchers to accurately simulate a wide variety of real-world processes and conditions. In Dr. Karanikola’s lab, QSensors were fabricated by coating the surface of quartz crystal sensors with a range of engineered sorbent materials.
Real-time Insights into PFAS Adsorption Kinetics Using QSense QCM-D
McKenna Dunmyer’s research in Dr. Karanikola’s lab focuses on engineered sorbent materials with the potential to treat PFAS-contaminated water, with particular attention to the kinetics of interactions between PFAS and different surface materials. “Many researchers conduct kinetics or adsorption experiments with PFAS using batch methods,” Dunmyer explains, “but these only provide specific data points, without revealing the continuous adsorption process or the behavior of the entire system.” She emphasizes that the data obtained from QCM-D experiments were crucial for her recently published comparative study, which assessed PFAS adsorption mechanisms using four different PFAS molecules on both organosilane-functionalized and unmodified surfaces.3
“QCM-D was invaluable in providing a comprehensive view of how the kinetics of these systems vary depending on the surface chemistries of the materials, in combination with the distinct characteristics of the PFAS molecules we studied.”
In addition to adsorption behavior and kinetics, the QCM-D data provided valuable insights into the characteristics of the PFAS film layer on the engineered sorbent surfaces. The researchers were able to determine whether the adsorbed layer was compact and rigid, or exhibited viscoelastic properties, which helped to confirm how the PFAS molecules were interacting with the surface. This information was critical in understanding how the molecular geometries of the PFAS influenced the behavior of the adsorption film, further refining their understanding of the adsorption mechanisms.
Translating QCM-D Data Into Better Remediation Strategies
McKenna Dunmyer’s QCM-D study revealed how electrostatic and hydrophobic interactions at sorbent material surfaces influence PFAS adsorption and clarified the effects of PFAS chain length and head groups on adsorption rates. Building on these findings, the research group plans to study a wider range of PFAS concentrations and optimize interfacial structures to improve water treatment methods. They also aim to use QCM-D to explore PFAS interactions with biomolecules, such as proteins, to quantify binding affinities and other attachment characteristics, contributing to public health guidance. While many questions about PFAS remain, scientists like Dr. Karanikola and McKenna Dunmyer are committed to deepening our understanding of these persistent contaminants. Their continued research, leveraging QCM-D technology for increasingly complex nanoscale experiments, promises further breakthroughs in PFAS remediation.
About the KORES Lab Group
The KORES Lab at the University of Arizona, led by Dr. Vicky Karanikola, specializes in advanced water and wastewater treatment, with a focus on optimizing processes and materials at the water-energy nexus. The lab combines fundamental research with applied solutions, addressing key challenges like energy efficiency and cost reduction while transitioning technologies from lab-scale to practical applications. Research efforts are guided by community water quality concerns, and the team collaborates with utilities on pilot-scale projects to validate water treatment and reuse technologies. A significant area of investigation includes developing targeted methods for the selective removal of emerging contaminants, such as PFAS.
References
- https://www.fda.gov/food/environmental-contaminants-food/and-polyfluoroalkyl-substances-pfas. ↩︎
- Al Amin, Md., et al. “Recent advances in the analysis of per- and polyfluoroalkyl substances (pfas)—a review.” Environmental Technology & Innovation, vol. 19, Aug. 2020, p. 100879, https://doi.org/10.1016/j.eti.2020.100879 ↩︎
- Dunmyer, McKenna, et al. “Molecular scale adsorption behavior of per- and poly-fluoroalkyl substances (PFAS) on model surfaces.” Chemical Engineering Journal, vol. 497, Oct. 2024, p. 154286, https://doi.org/10.1016/j.cej.2024.154286 ↩︎