Cryo-electron microscopy (cryo-EM) is a powerful imaging technique used to determine the structure of biological macromolecules, viruses, and cellular components at near-atomic resolution. Unlike traditional electron microscopy, which requires heavy metal staining or dehydration, cryo-EM preserves biological samples in their native hydrated state by rapidly freezing them.
The cryo-EM workflow encompasses three distinct steps: sample preparation, cryogenic imaging and image processing and reconstruction. The quality of the result achieved is dependent on all three steps. Sample preparation starts with purifying the sample of interest to obtain an aqueous solution of homogenous particles. If the purification process does not yield a sample of sufficient quality, all subsequent efforts would be wasted.
Negative stain EM plays a crucial evaluative step of the purified sample to determine if the sample meets a few basic criteria such as the size and shape of the particles, stability, concentration and its homogeneity1.

Negative Stain Electron Microscopy
Negative stain electron microscopy (EM) is a classical technique that enhances the contrast of nanoscale structures using electron-dense stains, allowing researchers to quickly visualize sample morphology and particle sizes2–3. Within the cryo-EM community, negative staining has long served as an essential preliminary screening step. It enables scientists to assess sample purity and homogeneity before committing to the more resource-intensive vitrification required for cryo-EM imaging. It also provides valuable preliminary information when preparing novel specimens.
Despite its historical significance, negative stain EM is increasingly being phased out in modern cryo-EM labs. In this evolving landscape, desktop STEM instruments like the Phenom Pharos offer a compelling alternative by providing rapid, high-contrast transmission imaging that integrates seamlessly into the workflow, effectively addressing bottlenecks in the cryo-EM workflow.
What Are Common Challenges Encountered When Using Negative Stain Electron Microscopy as a Screening Technique?
The main challenge for cryo-EM labs using negative stain EM is the reliance on traditional, often aging, room temperature TEM instruments. These systems introduce several issues when incorporated into sample preparation workflows:
- High cost and space requirements: Traditional TEMs are expensive, cost millions of dollars, and require dedicated rooms with specialized cooling and electrical infrastructure (Figure 1). Infrastructure and maintenance costs, which include sizable service contrast that cover the microscope and detectors, pose significant cost burdens on cryo-EM labs.
- Limited automation and ease-of-use: Conventional TEMs are predominantly manually operated, lacking the advanced automation features found in modern cryo-EM instruments. This results in prolonged training periods, often taking weeks or even months for novice users to achieve proficiency.
- Slow sample loading and setup time: Negative staining often involves testing multiple conditions and stains, generating numerous grids for imaging. Each grid must be individually loaded into TEM following by pump down and microscope alignment prior to imaging, leading to increased operational costs and time.
Recent trends indicate that many labs now consider the extensive time, cost, and space required to maintain a large TEM solely for negative staining to be an unjustifiable investment. Consequently, some are bypassing this critical preliminary screening step and transitioning directly from sample purification to cryo-EM. While this approach introduces additional risks and may result in higher costs when validating samples under cryogenic conditions, laboratories view it as a pragmatic tradeoff considering the limitations associated with traditional TEM workflows.
What Are the Benefits of Using a Desktop STEM to Screen Negative Stained Samples?
Recent technological breakthroughs have ushered in the era of compact scanning transmission electron microscopes (STEM), transforming how negative stain EM experiments are conducted. The Phenom Pharos Desktop STEM exemplifies this shift, integrating a high-brightness field-emission source with a fully integrated segmented STEM detector to produce high-contrast transmission images even at low voltages. This innovative system is highly effective at generating high-contrast transmission electron images, making it ideal for visualizing biological samples prepared with negative staining.
Here are the five key advantages of using the Phenom Pharos Desktop STEM for screening negative stained samples:
1. Fast Sample Loading and Rapid Imaging
Standard 3 mm TEM grids can be quickly and securely loaded into the sample chuck. The optimized stub features a robust copper clamp to ensure grid flatness and protection, while the fast pump down mechanism and intuitive interface allow imaging to begin in less than one minute. The video in Figure 2 demonstrates how easy this process is.
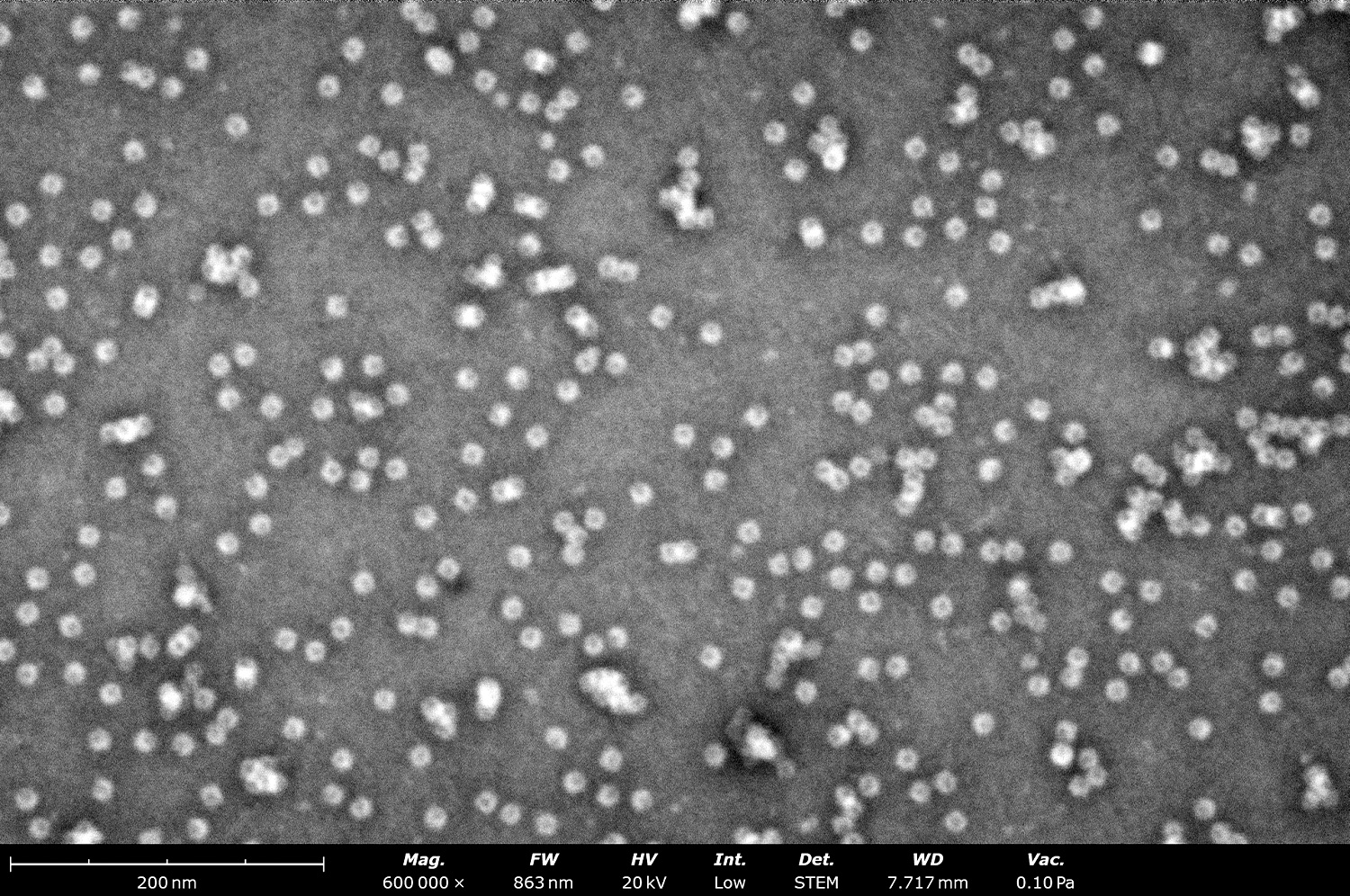
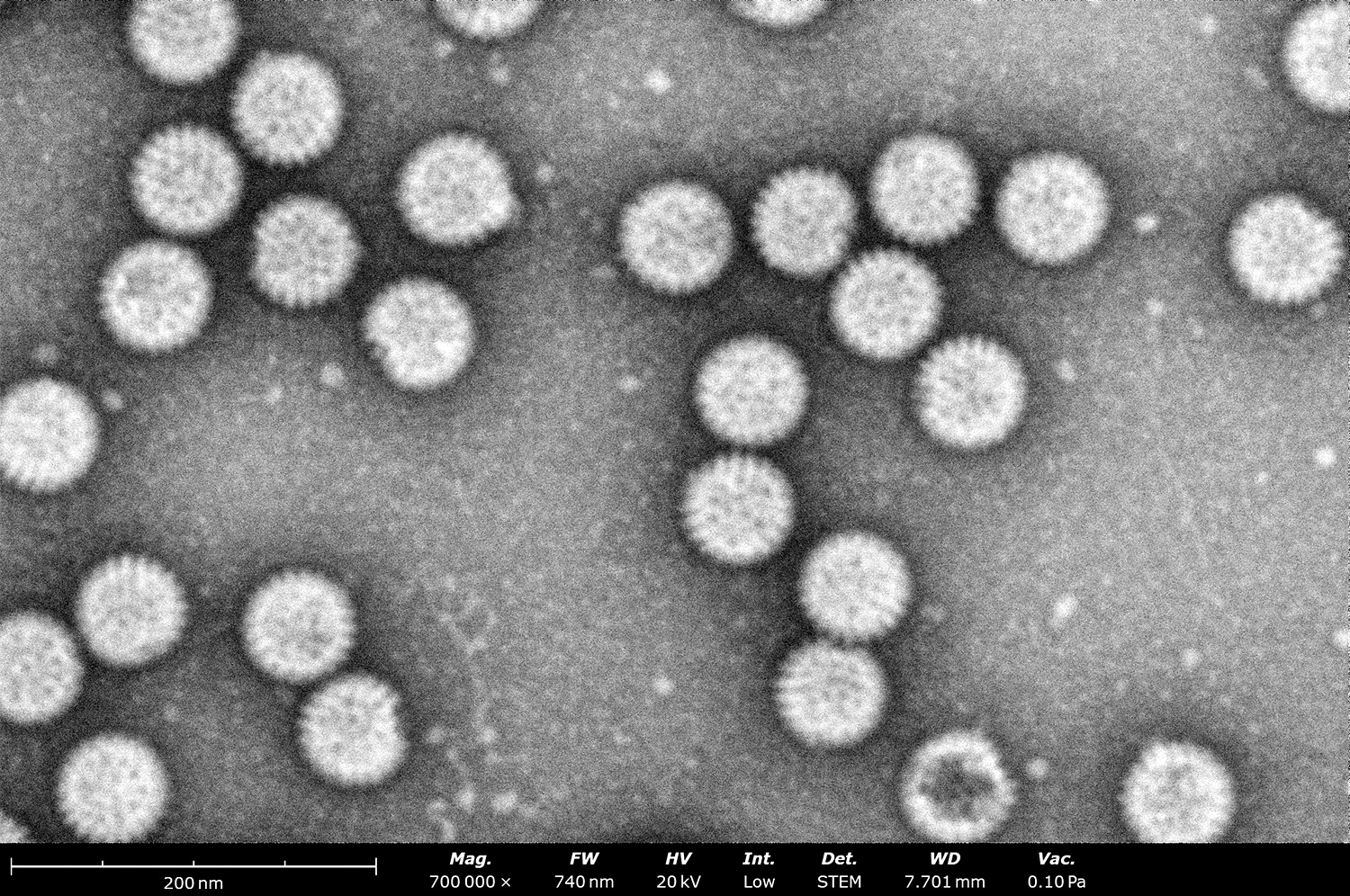
2. Effective Assessment of Sample Purity, Size, and Concentration
Although its resolution may not match that of conventional TEMs, the Phenom delivers images of negatively stained grids that adequately answer key questions: Is the sample present, intact, and pure? Does it match the expected size and morphology? Is the sample agglomerated? As seen in Figure 3, the quality of images produced by the Phenom Pharos Desktop STEM are sufficient for structural biologists to observe these key characteristics, greatly streamlining the overall cryo-EM workflow.
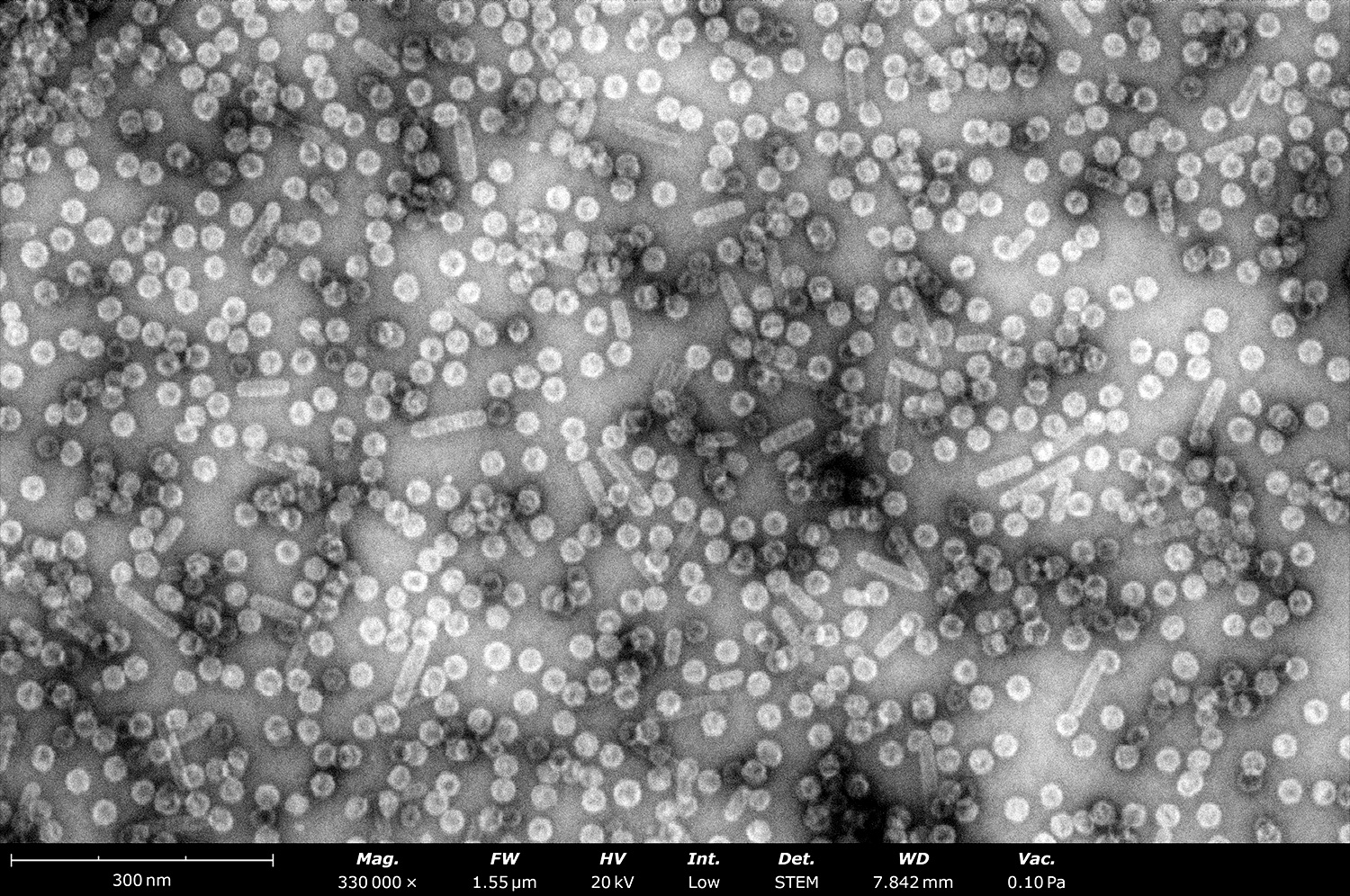
3. Expansive Imaging Area
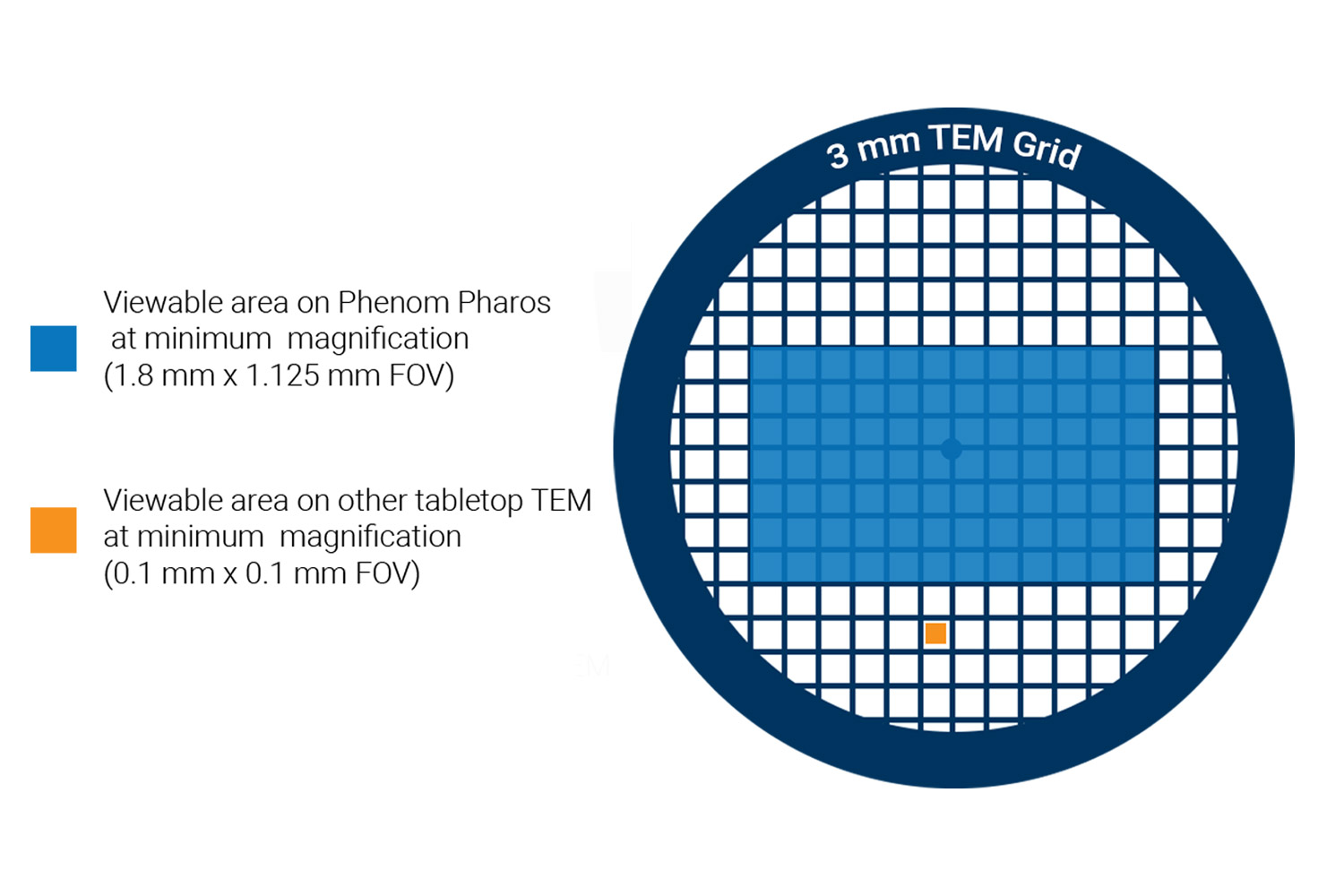
With a large pixel array detector, nearly the entire sample grid can be viewed at minimal magnification which provides up to 200 times more field of view than traditional TEM systems (Figure 4). This extensive coverage makes it an excellent tool for quickly assessing grid quality and identifying areas of interest.
4. Cost-Effectiveness
Designed with a user-friendly interface, stable electron optics, and easy sample loading, training new users can take as little as one day. The robust system and intuitive design make it much less prone to being damaged by novice users. Combined with the fast time-to-image, these factors support a cost-effective operation model that minimizes training time, unscheduled downtime, and the typical session time for screening negatively stained samples.
5. Compact Design and Small Footprint
The small footprint of the desktop STEM allows for flexible placement in a variety of laboratory settings (Figure 5), eliminating the need for dedicated, large-scale facilities with specific infrastructure requirements. This efficiency in space usage means that the lab area occupied by traditional TEMs can be reallocated for other critical equipment.
Conclusion
In summary, while negative stain EM has long played a pivotal role in screening and evaluating sample quality for cryo-EM, its traditional reliance on bulky, high-cost TEMs poses significant challenges. Desktop STEM systems like the Phenom Pharos not only address these limitations by offering rapid sample loading and imaging, user-friendly operation, and the resolution needed to evaluate critical sample characteristics but also deliver a cost-effective and space-efficient solution. This innovative approach enables labs to maintain the benefits of negative stain EM while streamlining workflows and optimizing resources. As research needs evolve, adopting these new tools promises a bright future for labs committed to conducting high-quality structural biology research.
To learn more about the Phenom Pharos Desktop STEM, contact us or check out our recent webinar, Enhancing Cryo-TEM Efficiency: Screening Negatively Stained Samples with Desktop STEM.
References
- C. A. Scarff, M. J. G. Fuller, R. F. Thompson and M. G. Iadaza, “Variations on Negative Stain Electron Microscopy Methods: Tools for Tackling Challenging Systems,” Journal of Visualized Experiments, vol. 132, p. 57199, 2018. ↩︎
- S. D. Carlo and J. R. Harris, “Negative staining and Cryo-negative Staining of Macromolecules and Viruses for TEM,” Micron, pp. 117-131, 2011. ↩︎
- J. R. Gallagher, A. J. Kim, N. M. Gulati and A. K. Harris, “Negative‐Stain Transmission Electron Microscopy of Molecular Complexes for Image Analysis by 2D Class Averaging,” Current Protocols in Microbiology, vol. 54, no. 1, p. e90, 2019. ↩︎