Introduction
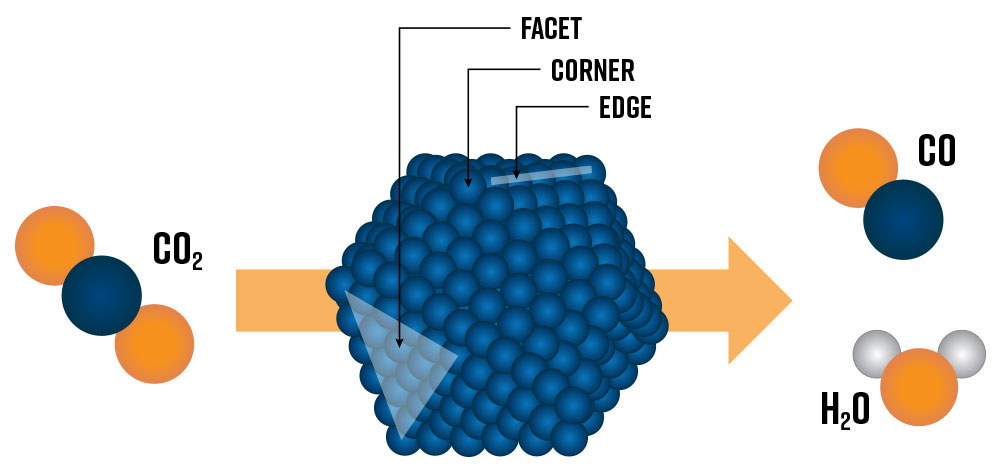
Heterogeneous catalysts are essential in today’s chemical industry and are crucial for enabling tomorrow’s green hydrogen economy [1]. Developing the next generation of heterogeneous catalysts of tomorrow requires a fundamental understanding of catalytic active sites at the nanoscale. Active sites lower the kinetic energy of reactions by providing a physical location for reactant species to absorb and desorb, speeding up the rate of reactions. Today’s state-of-the-art catalysts are nanostructured materials composed of metallic nanoparticles (e.g., Pt, Ru, Ni, Co) deposited on an oxide support (e.g., Al2O3, TiO2, SiO2). The metal nanoparticle’s size and shape have a profound impact on which types of potential active sites and surface structures are exposed to reactants and influence the overall catalytic performance of the system (Figure 1) [2]. Generally, smaller nanoparticles will have a higher fraction of surface metal atoms (e.g., facet, corner, and edge sites) which can lead to higher catalytic activity. However, below a certain threshold size, nanoparticles may no longer accommodate the most active sites for specific reactions.
To unravel the specific features of a metallic nanoparticle that direct its catalytic performance, extensive in situ and operando characterization methods are employed. This involves observing a so-called ‘model’ catalyst material under near-reaction conditions (i.e., in situ), and optionally measuring conversion (i.e., operando), using high-resolution transmission electron microscopy (e.g., AC-TEM, HAADF-STEM, STEM-EDX, STEM-EELS) and/or spectroscopy (e.g., in situ FTIR, XAS, etc.) techniques [3, 4, 5]. Model catalysts contain nanoparticles of well-defined size, surface facets, and compositions, allowing researchers to elucidate structure-activity relationships from experimental data and ultimately engineer more efficient and selective catalysts for industrial applications.
Nanoparticle Synthesis with Conventional Methods
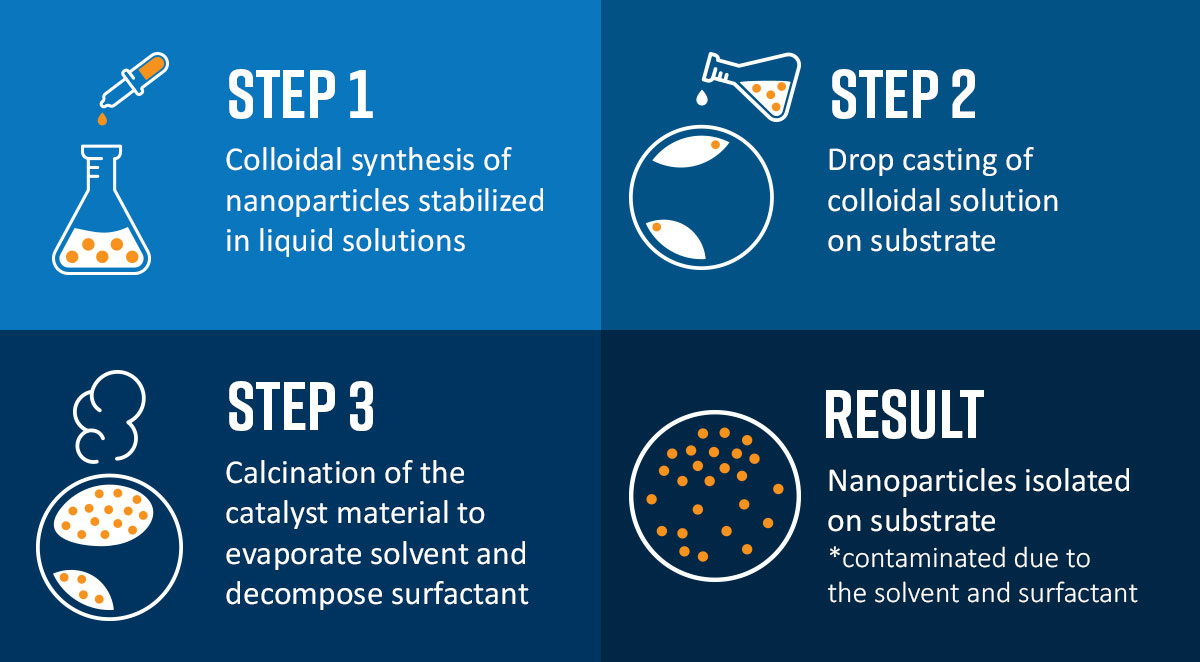
Conventional synthesis methods for creating heterogeneous catalyst nanoparticles involve bottom-up approaches such as co-precipitation, impregnation, colloidal synthesis (Figure 2), and hydrothermal synthesis to name a few.
All these strategies involve wet chemistry in which precursors are dissolved in a solvent and the resultant nanoparticles are formed in solution. Such approaches are generally not suitable for the preparation of model catalysts required for advanced microscopy or spectroscopy measurements because of the difficulty in completely removing residual surface contamination from the as-synthesized material [6]. Removing surface contamination via thermal treatment at high temperatures (> 500 °C) can cause the nanoparticles to sinter or damage the substrate on which the nanoparticles are deposited. Versatile methods to produce model unsupported and supported catalysts are therefore vital to improve our understanding of catalytic systems and develop future catalysts.
Spark Ablation: Top-down Fabrication of Pure Nanoparticles
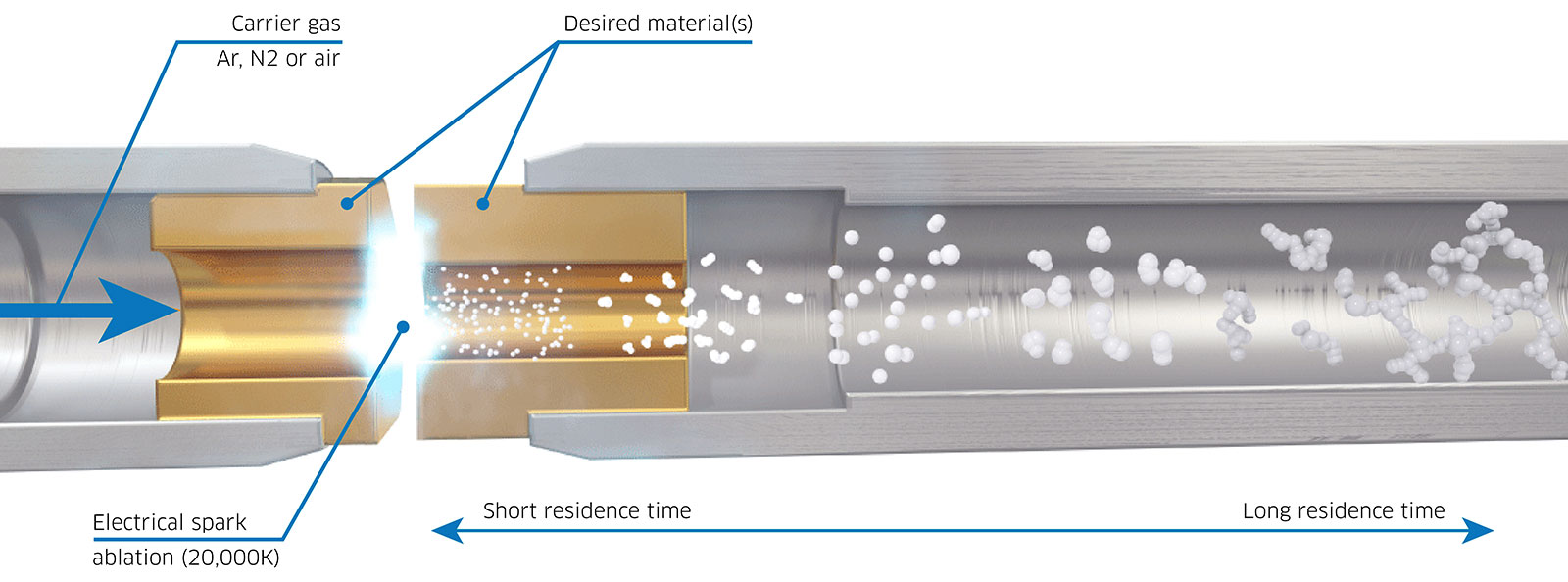
A promising substitute for the wet synthesis of nanoparticles is spark ablation. During spark ablation, a rapid, high-voltage pulse (lasting 1-10 μs) is applied across two electrodes of the metal(s) or semiconductor(s) of choice (Figure 3). As voltage is applied across the two electrodes, which act as a capacitor, a high-temperature spark (>20,000 K) is eventually discharged which causes ablation, generating an aerosol of pure particles. These particles can subsequently be transferred to a variety of downstream substrates using a flow of inert carrier gas. The nanoparticles produced through this method consist of pure metal, alloy, or semiconductor materials, eliminating the need for post-processing steps like drying or surfactant decomposition. Consequently, spark ablation emerges as a more straightforward approach to generating high-quality nanoparticles for use as model catalysts.
VSParticle’s spark ablation technology offers a versatile method to rapidly produce nanoparticles for today’s catalysis researchers. The VSP-G1 Nanoparticle Generator (VSP-G1) can produce tunable metallic nanoparticles at the push of a button.
It consists of the following basic parts (Figure 4):
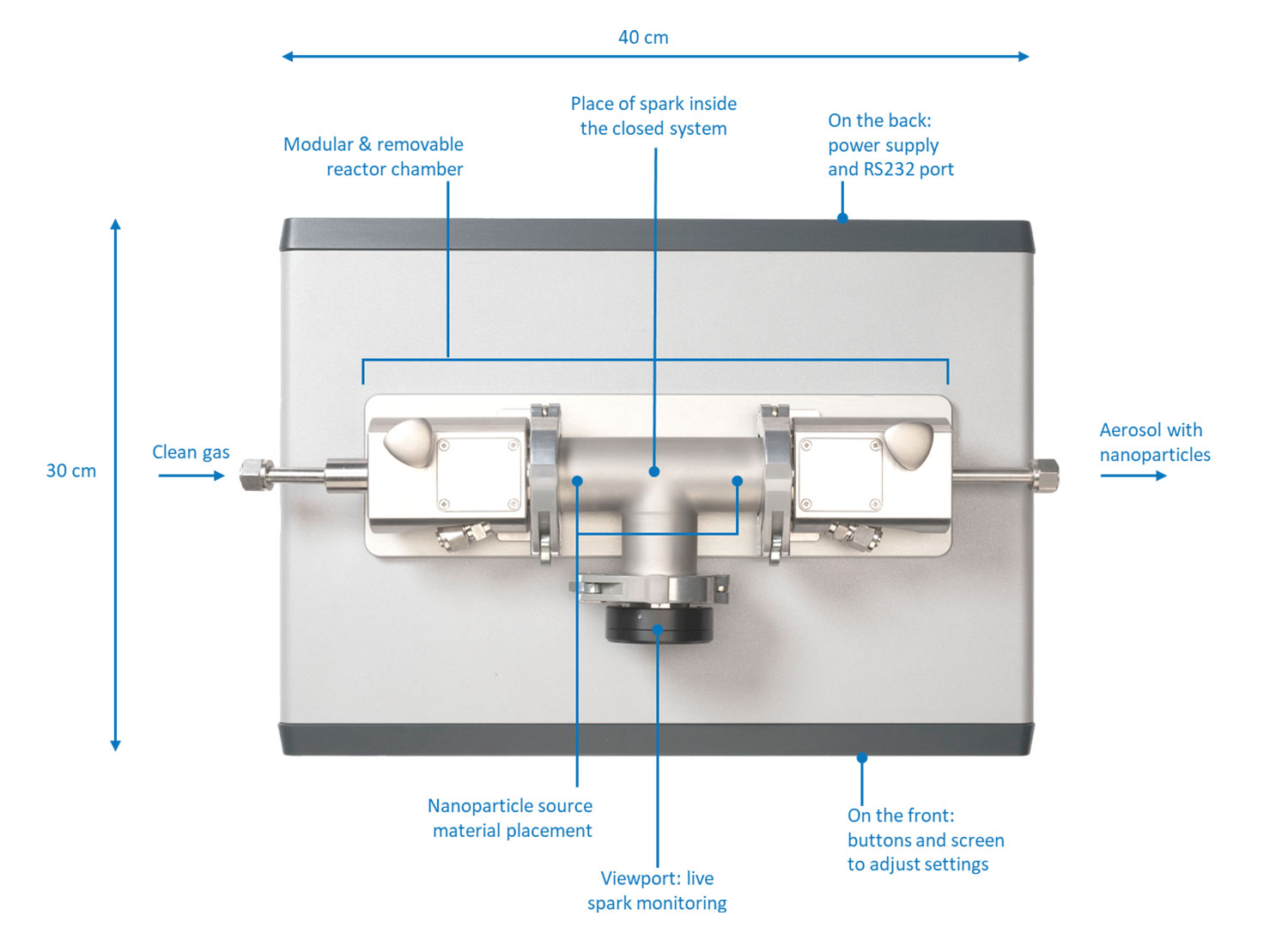
- Electrodes of the source material
- Modular and removable reactor chamber
- Carrier gas inlet and outlet
- Voltage source
- Optional: deposition accessories (VSP-A series) connected to the outlet
A compact footprint (40 by 30 cm) allows it to be placed on a laboratory bench and it can be operated directly through the front panel or remotely using the VSP-C1 controller unit. The controller unit is a touchscreen tablet that allows for all process parameters to be modified and monitored during the use of the instrument. The VSP-A series deposition accessories can be connected to the outlet to deposit the particles on a variety of substrates through diffusion (VSP-A1), filtration (VSP-A2), and impaction (VSP-A3).
Control of key particle characteristics, including the size (distribution), concentration, and coverage (on the downstream substrate) are dictated by the carrier gas flow rate, voltage, and current applied to the electrodes, and the amount of time spark discharge is enabled. Gas flow rate has a primary effect on the particle size and concentration before they are carried downstream wherein faster flow rates reduce the ability of particles to aggregate which results in smaller particles with a narrower size distribution. Higher flow rates also result in higher particle concentrations and substrate coverage.
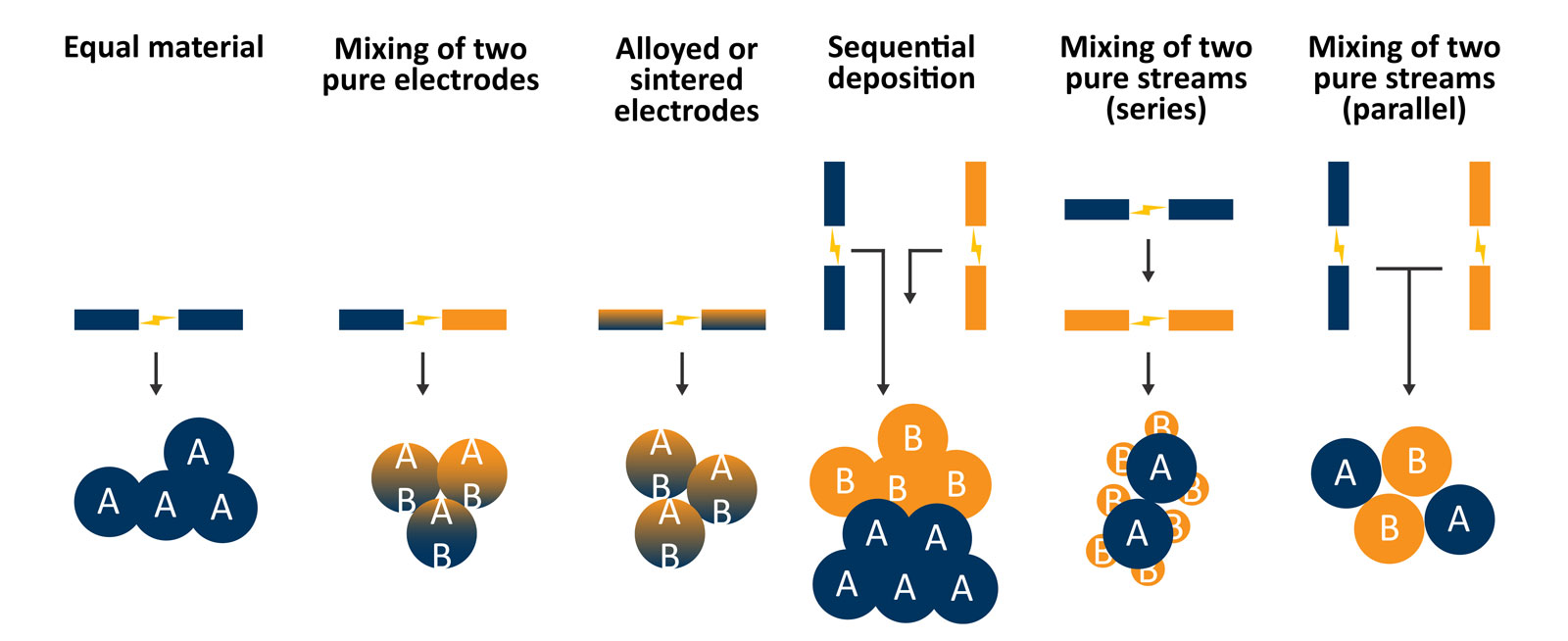
Another possibility that is advantageous for catalysis researchers is the ability to generate complex and hierarchically structured catalysts using the VSP-G1 (Figure 5). Using a single system and different electrode material combinations (e.g., equal materials, mixing of two pure electrodes, or alloyed or sintered electrodes) can be leveraged to modulate the individual particle composition. With two systems, either arranged in series or parallel and using two different types of electrode material in each spark generator, more complex structures can be created such as decorated supports.
Direct Preparation of Catalyst Nanoparticles for High-Resolution Electron Microscopy
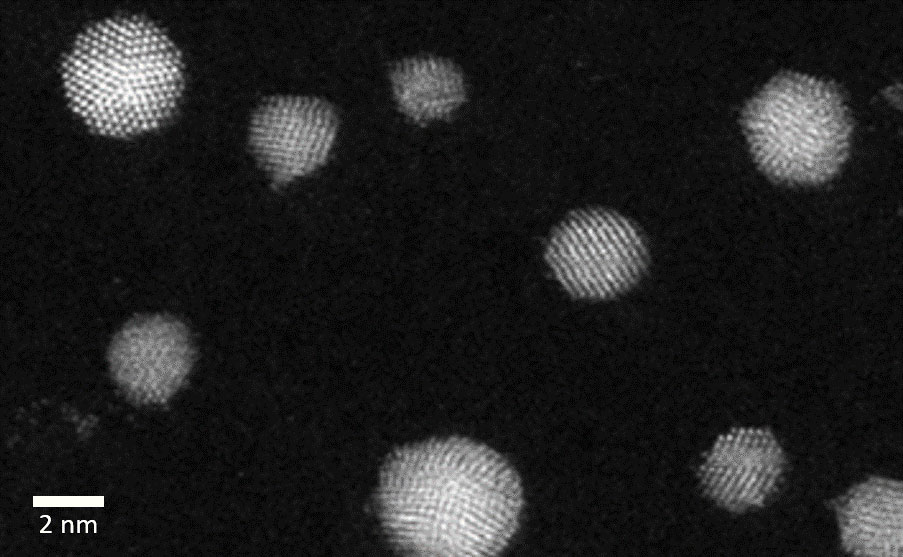
The VSP-G1 Nanoparticle Generator can be leveraged to quickly produce size-tuned metallic nanoparticles that are ready for advanced high-resolution electron microscopy studies such as in situ TEM. Surfactant-free nanoparticles can be deposited directly onto a TEM grid or 2D substrate using the VSP-A1 Diffusion Accessory. This approach was directly implemented to prepare unsupported Au nanoparticles in the size range of 2-5 nm on a TEM grid and imaged with high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) (Figure 6). These highly crystalline nanoparticles are free of stabilizing ligands/surfactants that can influence the nanoparticle shape, making these nanoparticles ideal for in situ TEM studies. In addition, by equipping the VSP-G1 with pre-alloyed electrodes, bimetallic/multi-metallic nanoparticles can also be produced and studied to unravel their unique catalytic properties.
Enabling In Situ Raman Spectroscopy Studies of Industrially Relevant Nickel Catalysts
Recent advances in many spectroscopic techniques have ensured that today’s highest-impact catalysis researchers have embraced in situ/operando spectroscopy in their works. For example, vibrational techniques such as Raman spectroscopy can provide insights into both the surface and gas-phase species that are participating in the catalytic reaction. To enhance the Raman signal and effectively study these reactions, Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy (SHINERS) employs catalytically active nanoparticles (e.g., Ni, Pt, Rh) deposited on SiO2 encapsulated Au nanoparticles (Au@SiO2) for in situ studies. The Shell-Isolated Nanoparticles (SHINS) have low thermal stability (ca. 450°C), therefore SHINERS studies have typically been limited to studying precious metal catalysts (e.g., Rh, Pt, Pd) that can be reduced at low temperatures to form metallic nanoparticles on the Au@SiO2. However, industrially relevant metals such as Ni are inactive for hydrogenation reactions unless a high-temperature reduction step (ca. 500 °C) precedes the catalytic reaction. Methods to produce SHINERS active catalysts with industrially relevant metals (e.g., Ni, Co, Cu, Fe) are therefore highly desired.
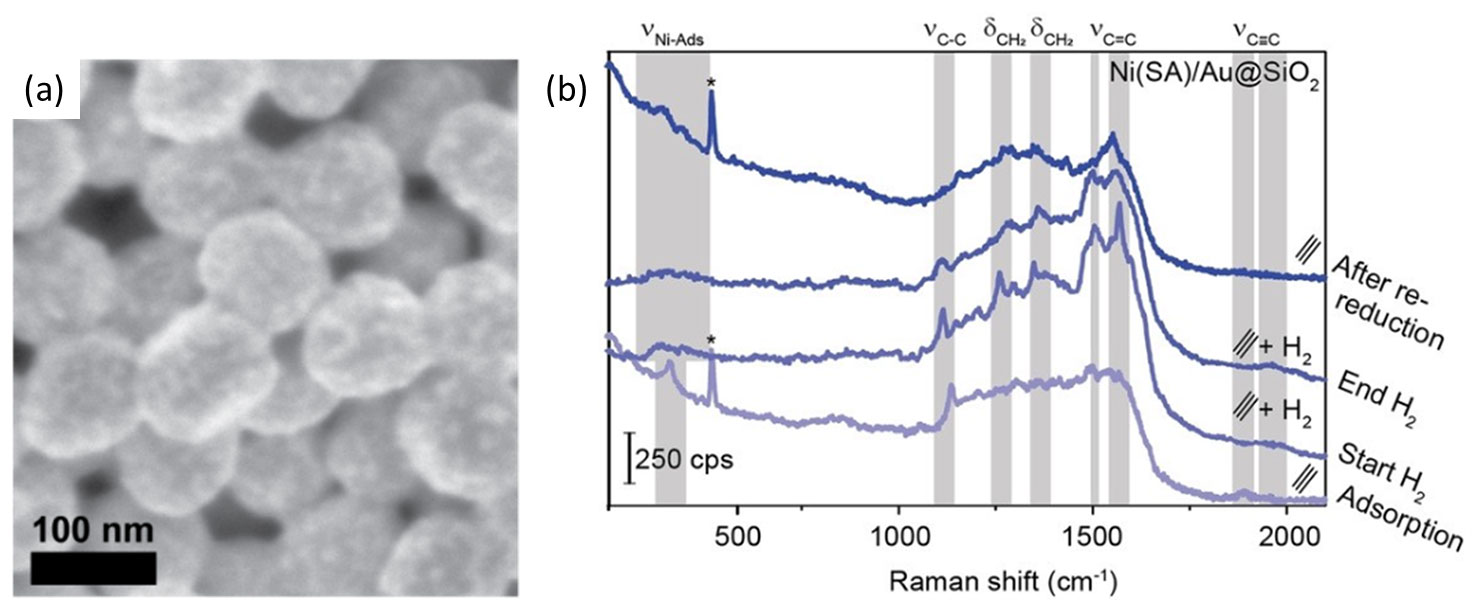
A recent publication by Wondergem et al. demonstrated how clean Ni nanoparticles were deposited on Au@SiO2 SHINS via spark ablation (Figure 7a) [7]. Compared with more conventional synthesis approaches such as impregnation and colloidal Ni nanoparticle deposition, spark ablation provided SHINERS-active catalysts. Importantly, these conventional wet synthesis methods required harsh thermal conditions to remove contaminants and activate the Ni-based catalyst. Ultimately such conditions destroyed the SHINS and were avoided by preparing Ni/Au@SiO2 via spark ablation. The spark ablation-prepared Ni/Au@SiO2 catalysts were employed for in situ Raman spectroscopy studies of industrially relevant hydrogenation reactions. The work by Wondergem et al. showcased how low transition metal catalysts can now be studied using SHINERS – a technique that was initially limited to precious metal-based catalysts. In Figure 7b, the spectra of Ni catalysts prepared via spark ablation using the VSP-G1 and VSP-A1 demonstrate this result. The spectra were obtained under acetylene hydrogenation conditions and highlighted the presence/absence of adsorbed surface species after exposure to the reactant gases and the formation of the gas-phase product during the in situ reaction. These results allowed the authors to determine which molecular species were present on the Ni surface during the Ni-catalyzed hydrogenation of acetylene.
Optimization of Electrocatalysts for Hydrogen Production
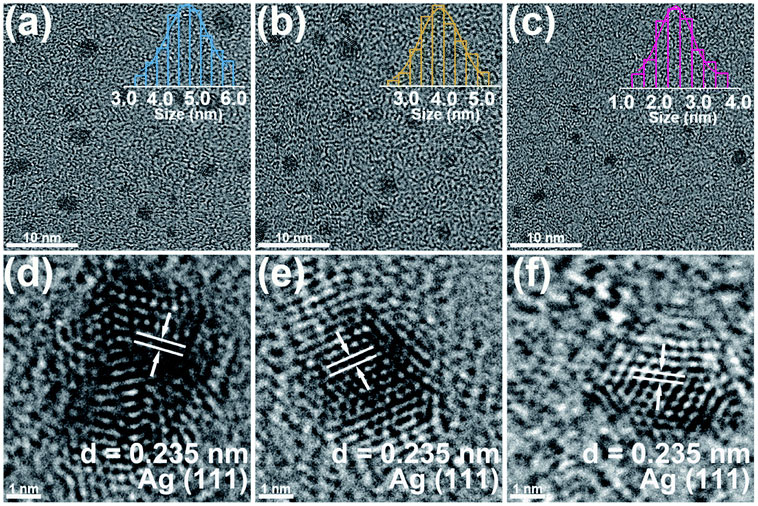
The ease of operation of the VSP-G1 Nanoparticle Generator allows for the systematic preparation of clean electrocatalysts, allowing for rapid screening and optimization of hydrogen evolution activity. This strategy was used by Lu et al. to evaluate the electrocatalytic hydrogen production and physical characteristics of silver nanoparticles deposited on carbon fiber substrates [8]. The carrier gas flow rate was varied from 5 to 25 mL/min which had an effect of decreasing the average particle size from about 4.7 nm to 2.4 nm as observed through TEM imaging (Figure 8) [8].
Furthermore, hydrogen evolution reaction (HER) polarization curves were acquired to evaluate the effects of carrier gas flow rate and total deposition time on performance, showing that the sample prepared at the highest flow rate (with correspondingly smallest average particle size) had an improved HER rate which was attributed to its higher surface area. Deposition time was optimal at 120 minutes, owing to reduced coverage at short deposition times and increased agglomeration (reduced surface area) at longer times. The authors noted that spark ablation has further application in the commercialization and mass production of electrocatalysts due to its relatively short processing time and ability to precisely control particle characteristics.
Conclusion
The VSP-G1 Nanoparticle Generator empowers researchers to conduct fundamental studies on model catalysts by providing a method to generate clean nanoparticles with precise control over particle size distribution. By relying on a completely dry process, namely spark ablation, the produced nanoparticles are free of residual surface contamination thereby eliminating the need for thermal post-processing that can cause particle coarsening and obscure surface active sites. This technology has already been demonstrated as a solution for directly preparing in situ TEM samples of gold nanoparticles and for preparing SHINERS-active samples for performing adsorption studies on industrially relevant nickel catalysts. The tool’s ease-of-use lends itself to rapid preparation of nanoparticles which can be exploited for systematic studies of electrocatalyst structure-activity relationships.
References
[1] D. Wei, X. Shi, R. Qu, K. Junge, H. Junge and M. Beller, “Toward a Hydrogen Economy: Development of Heterogeneous Catalysts for Chemical Hydrogen Storage and Release Reactions,” ACS Energy Letters, vol. 7, no. 10, pp. 3734-3752, 2022.
[2] R. A. V. Santen, “Complementary Structure Sensitive and Insensitive Catalytic Relationships,” Accounts of Chemical Research, vol. 42, no. 1, pp. 57-66, 2009.
[3] B. He, D. Y. Zhang, P. X. Liu and P. L. Chen, “In-situ Transmission Electron Microscope Techniques for Heterogeneous Catalysis,” ChemCatChem, vol. 12, no. 7, pp. 1853-1872, 2020.
[4] T. Altantzis, I. Lobato, A. D. Backer, A. Béché, S. B. Y. Zhang, M. Porcu, Q. Xu, A. Sánchez-Iglesias, L. M. Liz-Marzán, G. V. Tendeloo, S. V. Aert and S. Bals, “Three-Dimensional Quantification of the Facet Evolution of Pt Nanoparticles in a Variable Gaseous Environment,” Nano Letters, vol. 19, no. 1, pp. 477-481, 2019.
[5] T. Hartman, R. G. Geitenbeek, C. S. Wondergem, W. v. d. Stam and B. M. Weckhuysen, ” Operando Nanoscale Sensors in Catalysis: All Eyes on Catalyst Particles,” ACS Nano, vol. 14, no. 4, pp. 3725-3735, 2020.
[6] M. Cargnello, “Colloidal Nanocrystals as Building Blocks for Well-Defined Heterogeneous Catalysts,” Chemistry of Materials, vol. 31, no. 3, pp. 576-596, 2019.
[7] C. S. Wondergem, J. J. G. Kromwijk, M. Slagter, W. L. Vrijburg, E. J. M. Hensen, M. Monai, C. Vogt and B. M. Weckhuysen, “In Situ Shell‐Isolated Nanoparticle‐Enhanced Raman Spectroscopy of Nickel‐Catalyzed Hydrogenation Reactions,” ChemPhysChem, vol. 21, no. 7, pp. 625-632, 2020.
[8] J. Lu, J. Guo, S. Song, G. Yu, H. Liu, X. Yang and Z. Lu, “Preparation of Ag nanoparticles by spark ablation in gas as catalysts for electrocatalytic hydrogen production,” RSC Advances, vol. 10, pp. 38583-38587, 2020.