Improving Cell Infiltration in Electrospun Nanofibers
It is common knowledge that in order to biomimic the extracellular matrix (ECM) structure, electrospun fibers can be created with fiber diameter ranging in the nano- to micro-scale. Meanwhile, cell infiltration into scaffolding material is usually limited by pore size in its structure. In order to address this mishap, the scientific community has worked with various methods to increase pore size and porosity. Some of these include co-electrospinning fibers in the nano- and micro-scale at the same time, incorporating salt particles during fiber formation to eventually leach them out, co-electrospinning a water and non-water soluble fibers, and even the introduction of channels into the spun structure with laser ablation. Although these can successfully render large pores into the fibrous scaffold, limitations on reproducibility and homogeneity can still be a disadvantage.
When cells are cultivated in electrospun scaffolds, their viability is usually 100 µm in thickness due to limited oxygen and nutrient transport. In order to quickly increase cell density on the electrospun scaffold, the fabrication of tissue constructs based on the simultaneous electrospun fibers and electrosprayed cells has become more commonly used in tissue engineering and regenerative medicine. The image below shows a diagram of how electrospun fibers are simultaneously created with electrosprayed cells.
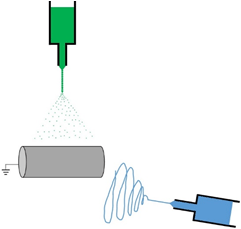
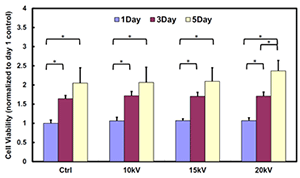
Electrospraying Cells into Electrospun Fibers: Cell Viability After Voltage Induction
It has been widely demonstrated that the survival rate of various cells that underwent high voltages during the electrospray process is higher than 95 %. By simultaneously electrospraying cells into electrospun fibers, cell viability and incorporation at different sample depth can be improved by co-depositing at the same time. A bar plot demonstrating the viability of cardiosphere-derived cells (CDCs) electrosprayed at different voltages (10, 15 and 20 kV) and cultured up to 5 days proves similar proliferation results to the non-exposed at high voltages.
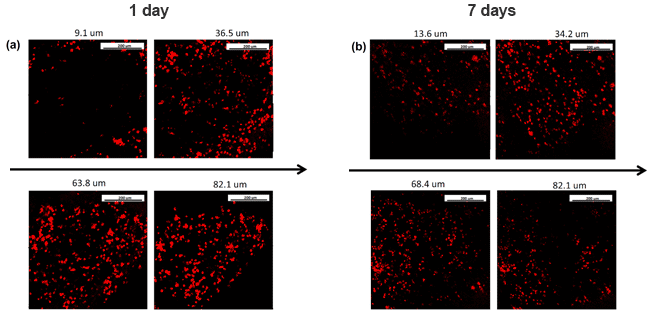
The following image shows the distribution of CDCs that were electrosprayed simultaneously with electrospun material that is able to mimic the cardiac ECM structure. It is shown that at different thicknesses cell distribution is homogeneous and viable after 1 (a) and 7 (b) days of culture. The Fluidnatek® equipment not only allows the simultaneous formation of electrospun fibers and electrosprayed cells, it is also able to scale up this process with its industrial fabrication equipment. With the combination of an environmental control unit, multi-axial capabilities, ultraclean working conditions by implementing a HEPA or ULPA filters, and UV-C lamp to maintain sterile conditions, these unit are able to maximize and become the ultimate versatile package when tissue engineering and regenerative medicine applications need to be employed.
References:
- 1 Yanyi Xu, et. al., Cardiac Differentiation of cardiosphere-derived cells in scaffolds mimicking morphology of the cardiac extracellular matrix, Acta Biomaterialia, 10, 2014, 3449-3462.
