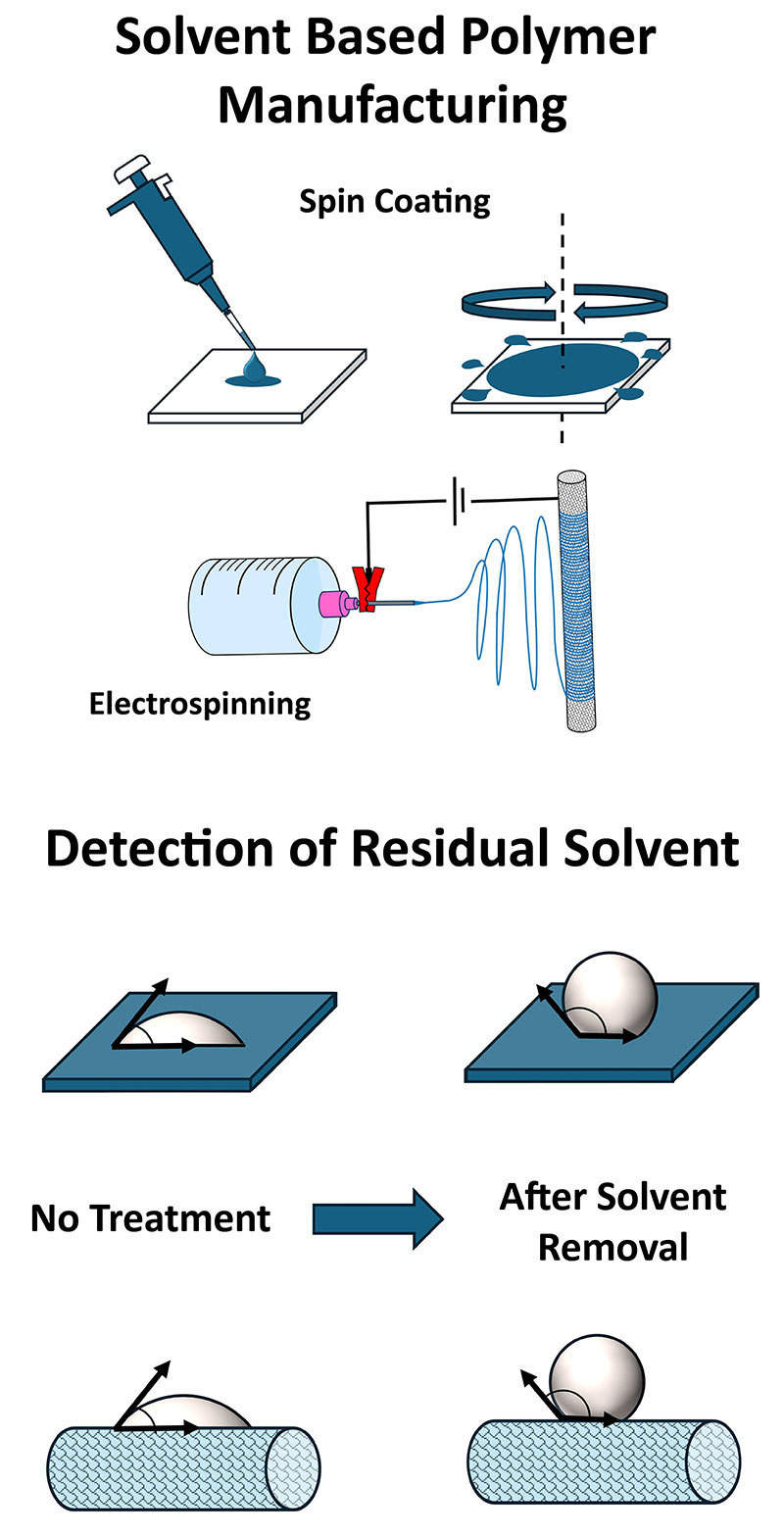
Introduction
Thin polymer films, membranes, and coatings are vital for functionalizing surfaces, as a protection layer, filtering chemicals, or tuning a material’s surface interactions. When fabricating thin polymeric layers, organic solvents are used to dissolve the polymer to reach a low enough viscosity or a specific phase for processing. This is common with thin polymer films formed with spin coating, porous polymer membranes made with non-solvent induced phase separation (NIPS), and polymeric nanofibers and particles fabricated with spray coating techniques like electrospinning/spraying. Ideally, the solvent is then removed amid manufacturing or with post-processing conditions. However, the need and effectiveness of post-processing to remove residual solvents are not always known or verified.
Polymer membranes developed with NIPS are used for water filtration [1] and electrospun fibers/particles are used as coatings for medicinal applications [2]. Residual solvents in these materials can have direct negative health effects since many of the solvents that are used are toxic to humans and the environment. Additionally, the presence of residual solvent can alter the crystalline phases [3] and mechanical properties of a polymer [2]. Therefore, techniques that can detect the presence of residual solvents rapidly and easily, can aid in safer and more efficient polymer manufacturing processes of products that rely on organic solvents. One technique that fulfills these requirements and can be readily coupled with polymer manufacturing is measuring water contact angle using optical tensiometry.
Solving the Solvent Problem
Detection and removal of residual solvents in polymer manufacturing processes may not be a trivial procedure. Detecting the presence of residual solvents should ideally be a quick and easily implemented method that is sensitive to a wide variety of solvents and polymer surfaces. While methods like mass spectrometry and thermogravimetric techniques are sensitive and quantitative, they can be challenging to implement, are destructive, and rely on expensive instrumentation and specialized works flows. Likewise, Fourier transform infrared (FTIR) absorption spectroscopy can be effective at detecting relative quantities of solvents but requires expertise for in-depth spectral analysis for each new solvent/polymer surface pairing. Alternatively, water contact angle measurements with an optical tensiometer can be an extremely sensitive, easy-to-operate, nondestructive, and inexpensive solution for detecting residual solvents in polymeric surfaces.
Measuring Water Contact Angles
Water contact angles are the angles made by a droplet of water on a surface of interest. The resulting angle is a function of the water’s surface tension, the solid’s surface energy, and the interfacial tension between the water and the solid. Using water as the probe liquid, surfaces that have high surface-free energy, like metals, oxides, glasses, and ceramics, are highly wettable and have a low contact angle. Conversely, surfaces with low surface energy, like many polymer surfaces, have limited wettability with water and therefore a high contact angle.
For a polymer film containing residual solvent, both the surface’s energy and intermolecular interactions between water and the sample surface are significantly altered compared to the pure polymer. Generally, the presence of residual solvent on a polymer surface will lower the contact angle. Using an optical tensiometer like the Theta Flow and the OneAttension software, we can quantify the change in contact angle of a polymer surface and quickly identify the presence and effective treatments for the removal of residual solvents.
To demonstrate this method, we tested the presence of residual solvents in two different manufacturing processes, spin coating and electrospinning.
Thin Films with Spin Coating
When making thin polymer films, spin coating is a versatile and widely used method. A flat sample is placed onto a rotational stage and a small quantity of polymer solution is placed on it. The sample is then spun at a specified RPM. The centripetal force of the rotation along with the surface tension of the liquid results in a thin film coating of the polymer on the sample. Depending on the solution properties, like viscosity, surface tension, and solvent volatility, and the rotation parameters, like spin speed, acceleration, and spin time, different thicknesses of polymer films can be achieved. A wide variety of organic solvents can be used to achieve different final film thicknesses. Solvents that are relatively volatile tend to evaporate entirely during the spin-coating process. However, solvents with lower vapor pressures and high boiling points can remain, requiring additional drying steps to remove fully.
The Power of Electrospinning
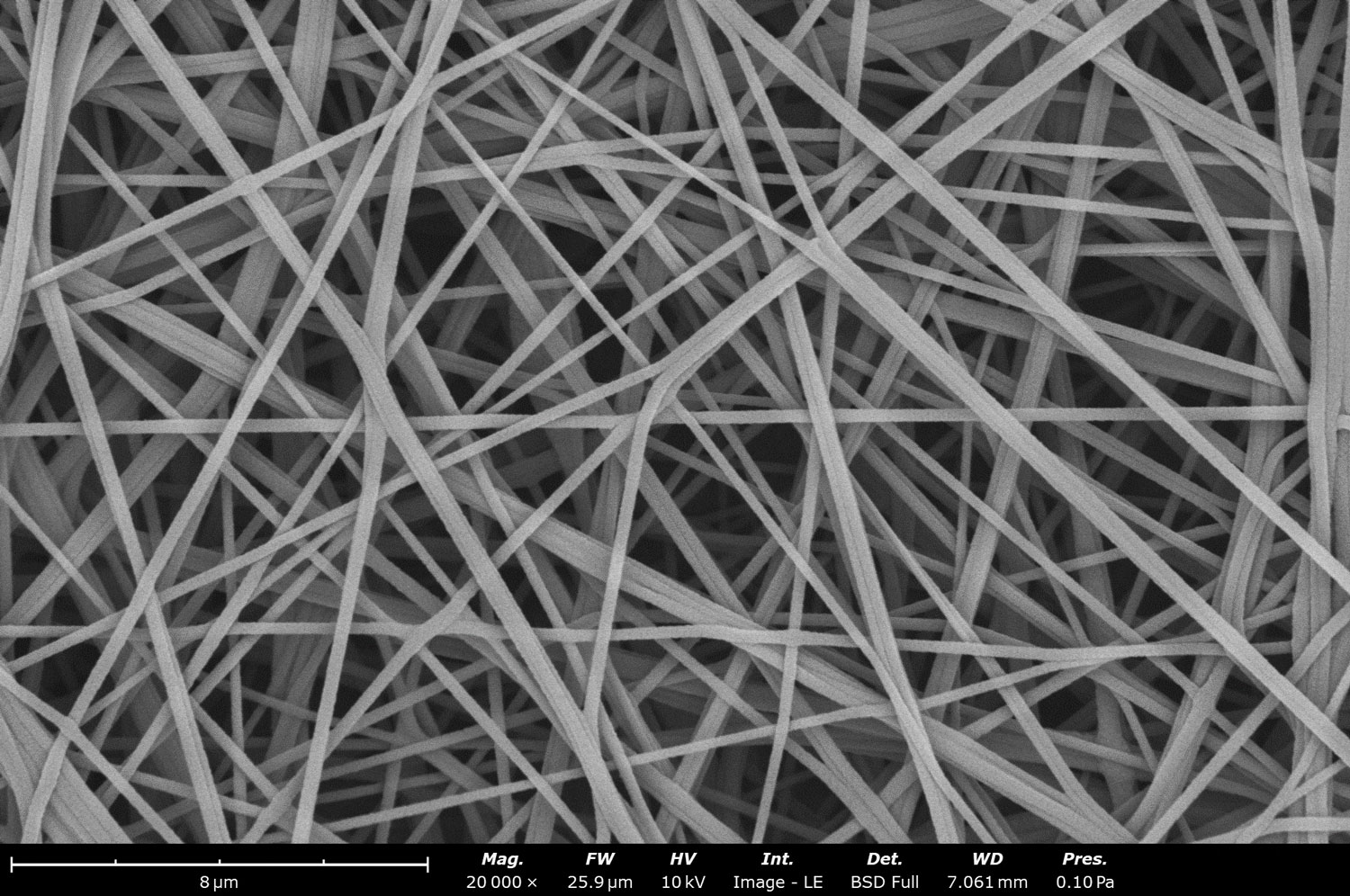
Electrospinning is a unique process that allows virtually any polymer in solution, emulsion, or suspension to be “spun” into nanofibers of varied sizes, ranging from tens of nanometers to microns in diameter. Figure 1 shows an SEM image of polymeric fibers fabricated with electrospinning. During electrospinning, a large voltage bias is applied to a needle tip filled with a solution of polymer inside a syringe. As the polymer solution is ejected from the needle in a thin stream, the solvent evaporates and the resultant fibers are collected on a grounded or oppositely biased substrate. The properties of the fibers are tuned by the applied voltage, polymer concentration, temperature, and a few other principal factors.
During the electrospinning process, the solvent is ideally completely evaporated during the fiber’s flight, but some residual solvent may remain in the polymer film. This can be the result of non-optimized processing conditions (temperature/humidity, solvent flow rate, needle-to-collector distance, voltage, or rotational speed), the intrinsic polymer-solvent affinity, or the boiling point/vapor pressure of the solvent. A high-quality electrospinning machine can provide full control of the processing conditions to significantly control this but will require additional testing to ensure complete solvent removal or if additional post-processing is needed.
Residual Solvent in Spin-Coated Films and Electrospun Fibers
To demonstrate water contact angle analysis for the detection of solvent residuals on polymer surfaces, we used the Theta Flow Optical Tensiometer equipped with the OneAttension measurement and analysis software. Water droplets of 3-5 µl were placed onto different polymer surfaces for up to 60 seconds while images of the droplet were recorded and subsequently analyzed to calculate the contact angle. This was done with two spin-coated films and an electrospun fiber mat. For the spin-coated films, two different polymer/solvent systems were studied: polyvinylidene fluoride (PVDF) dissolved in dimethylacetamide (DMAc) and polystyrene (PS) dissolved in toluene.
PVDF is a fluoropolymer that is used in numerous applications, including semiconductor and lithium-ion batteries. PVDF thin films made by spin-coating are typically derived from the polymer dissolved in a polar solvent with relatively low volatility like DMAc. Thus, it is very likely that spin-coated PVDF films will have residual solvent if no additional treatment is carried out. Contrarily, polymer films spin-coated with solvents like toluene, a non-polar and more volatile solvent, may not need additional treatment after spin-coating.
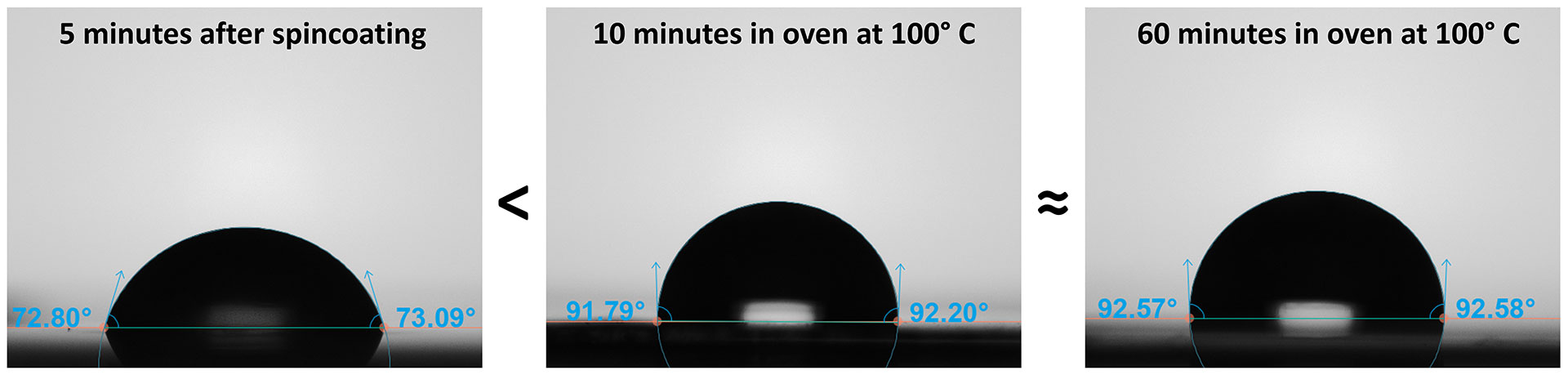
A UV/Ozone cleaned glass cover slip was spun-coated with PVDF in 20 wt % PVDF in DMAc. Within 5 minutes of the coating process, the water contact angle was measured and found to be 70.9 ± 1.0°. The PVDF film was then treated by drying in an oven at 100°C for either 10 or 60 minutes. Following the short and extended heat treatment, the water contact angles were measured to be 90.3 ± 1.5° and 90.9 ± 1.5°, respectively (Figure 2). These results reveal that heating the sample significantly increased the contact angle (+20°) due to the removal of residual solvent. But after 10 minutes in the oven, the lack of change in the WCA also indicates that all the solvent was removed within these first 10 minutes; any additional heating was superfluous. Therefore, the contact angle results provide evidence that heat treatment is necessary to remove the residual DMAc, but this can happen in a relatively short window at 100°C.
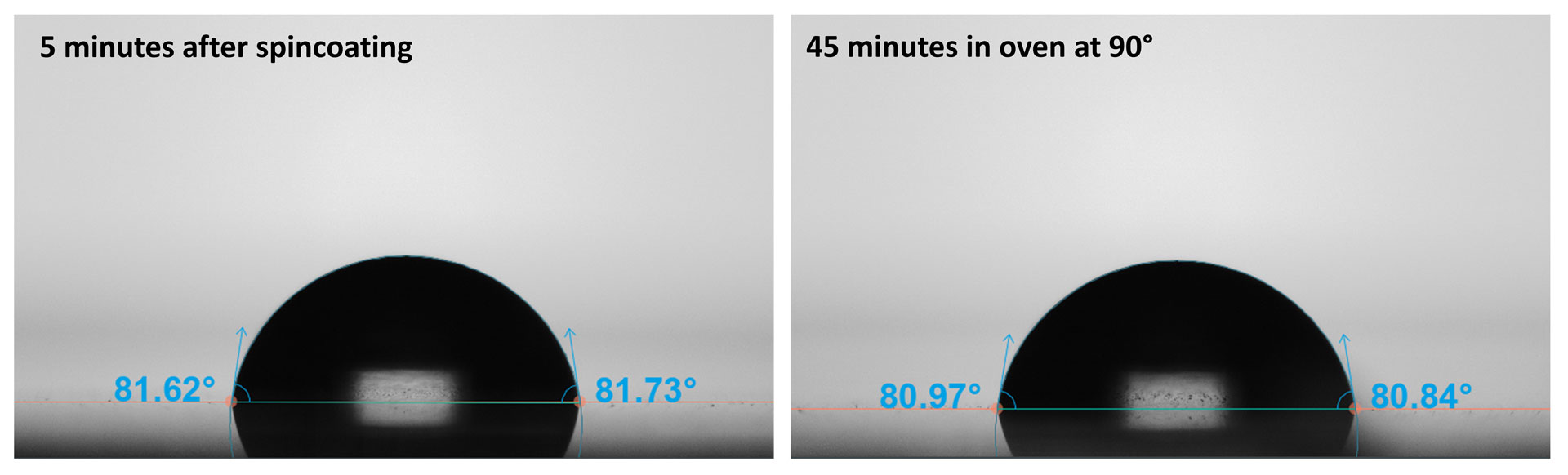
Next, a spin-coated film of PS derived from a 10 wt% solution in toluene was probed with water droplets before and after heating at 90°C for 45 minutes (Figure 3). The resulting contact angles with and without heat treatment were respectively 81.9 ± 0.2° and 80.6 ± 0.4°. The lack of change in contact angle tells us that no toluene lingered after the spin-coating process and was fully evaporated, suggesting any additional treatments to remove solvent are unnecessary.
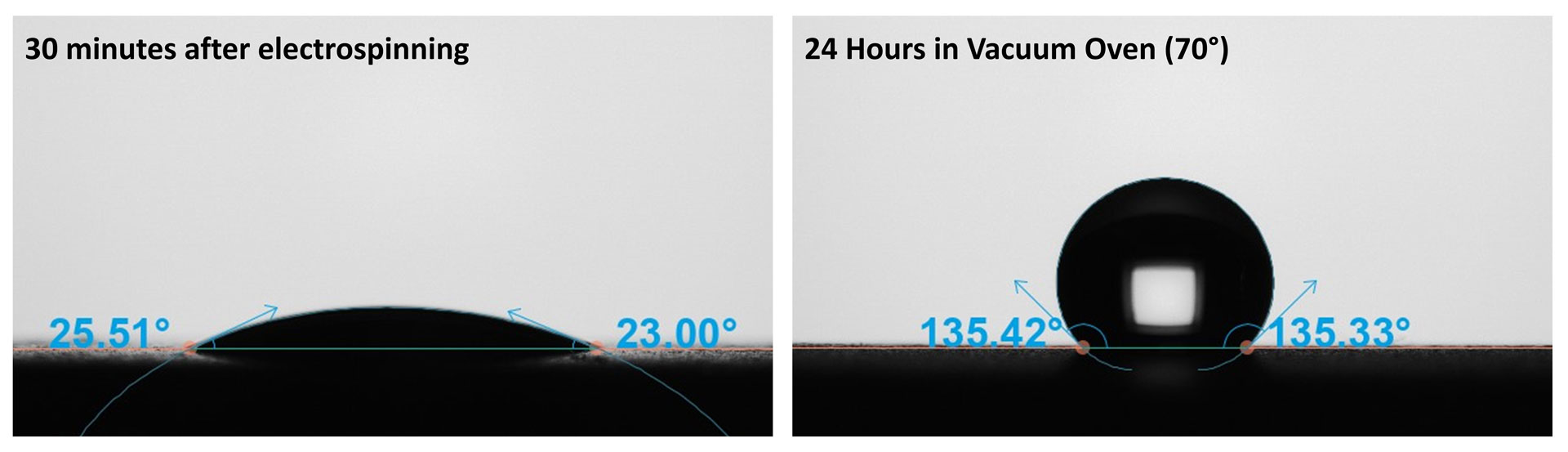
To highlight the versatility of this technique, water contact angle measurements were also performed on electrospun nanofibers derived from polyacryonitrile (PAN) dissolved in dimethyl sulfoxide (DMSO). PAN is commonly used in a wide variety of biomedical applications due to its ideal properties like light/chemical resistance, non-toxicity, mechanical robustness, and ease of electrospinning [5]. The fibers were generated with a Fluidnatek® LE-50 electrospinner with a 6% PAN in DMSO and an applied voltage of +8.8 kV and -1 kV on the needle and the collector, respectively. The polymer was electrospun at a needle-to-collector distance of 10 cm, using a flow rate of 1 mL/h, and maintaining the chamber at a temperature of 25 ± 1°C and relative humidity of 35 ± 5%. An SEM image (Figure 1) of the PAN fibers shows fibers with ~200 nm diameter with some bundling. The presence of bundles indicates that the electrospinning conditions and the solvent/polymer pair may not have been optimized for collecting dry fibers. A section of the fiber mat was taken for immediate contact angle analysis, while the rest of the mat was placed into a vacuum oven held at 70°C and 30 kPa for 24 hours. Higher temperatures were avoided to prevent glass transition in the PAN polymer or any other changes in morphology.
Figure 4 shows the resulting contact angle images, with large differences in the average contact angle values. The untreated sample (Figure 4, left image) had a water contact angle was 24.5 ± 1.0°. From this contact angle value, the PAN fiber mat is considered very hydrophilic. After the vacuum oven treatment (Figure 4, right image), the contact angle increased substantially to an average of 136.2 ± 1.1°. This indicates that the PAN is hydrophobic. The presence of DMSO made the polymer considerably more susceptible to wetting. As a control, the untreated PAN mat was also tested after sitting out for 24 hours at room temperature and pressure. Interestingly, the average contact angle increased to 40.3°, demonstrating that some solvent had evaporated, but a significant portion remained. It may be that further drying at room temperature is possible for additional solvent removal, but this may take a significant period due to the high boiling point and low vapor pressure of DMSO. Interestingly, if an aqueous-based coating step is required, the presence of residual DMSO may aid in spreading the coating or additives into the fiber mat.
| Material | Process | Treatment | WCA |
| PVDF in DMac | Spincoating | Ambient; 5 minutes | 70.9 ± 1.0° |
| 100°; 10 minutes | 90.3 ± 1.5° | ||
| 100°; 60 minutes | 90.9 ± 1.5° | ||
| PS in Toluene | Spincoating | Ambient; 5 minutes | 81.9 ± 0.2° |
| 90°; 45 minutes | 80.6 ± 0.4° | ||
| PAN in DMSO | Electrospinning | Ambient; 30 minutes | 24.5 ± 1.0° |
| Ambient; 24 hours | 40.3 ± 3.2° | ||
| 70°; 30 kPa; 24 hours | 136.2 ± 1.1° |
Table 1. Summarized water contact angle results on all the polymer film with and without solvent removal treatments.
Limitations of Water Contact Angle for Detecting Residual Solvent
While water contact angle measurements are a great tool for determining a surface’s wettability and residual solvent from manufacturing with polymers, there are some existing limitations. The first is that it does not quantify the amount of solvent removed or that remains. This would require a technique like TGA. But, TGA data could potentially be correlated to WCA, and WCA measurements used as a faster or routine QC analysis. Another potential drawback of this technique is that it can be influenced by morphological changes of the sample surface. If the treatment to remove solvent or post-process the sample changes the roughness of the surface, the contact angle can be affected. The treatments chosen for the spin-coated and electrospun surfaces were chosen to avoid melting or glass transitions of the polymers and retain their surface structure. Finally, while WCA measurements are generally non-destructive, if a sample surface is sensitive to water damage or can be dissolved by water, this technique may want to be avoided.
Conclusion
Water contact angle measurement taken with a high-quality optical tensiometer is a powerful technique to detect the presence of residual solvent on polymer surfaces and determine effective removal treatments. This can be applied to virtually any solvent-based polymer manufacturing process. This successfully detected the presence of residual DMAc in spin-coated PVDF films, the lack of toluene in spin-coated PS films, and the presence of significant DMSO in electrospun PAN fibers. Preventing residual solvent is important to maintaining the mechanical/physical properties of polymers, tuning interfacial properties like wettability and biofilm adhesion, and inhibiting negative health or environmental effects when using toxic solvents.
References
[1] Dong X., Lu D., Harris T.A.L., Escobar I.C., “Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development.” Membranes, vol. 11, no. 5 pp 309, Apr. 2021.
[2] D’Amato A. R., Bramson M. T. K., Puhl D. L., Johnson J., Corr D. T., Gilbert R. J. “Solvent retention in electrospun fibers affects scaffold mechanical properties.” Electrospinning, vol. 2, no. 1, pp 15-28, Feb. 2018.
[3] Nishiyama T., Sumihara T., Sato E. et al., “Effect of solvents on the crystal formation of poly(vinylidene fluoride) film prepared by a spin-coating process.” Polym J, vol. 49, pp 319–325, Mar. 2017.
[4] Nam J., Huang Y., Agarwal S., Lannutti J., “Materials selection and residual solvent retention in biodegradable electrospun fibers.” J. Appl. Polym. Sci, vol. 107, pp 1547–1554, Oct. 2007.
[5] Sarwar, M. N. et al. “Evaluating antibacterial efficacy and biocompatibility of pan nanofibers loaded with diclofenac sodium salt.” Polymers, vol. 13, no. 4, pp 510, Feb. 2021.