Amid the COVID-19 pandemic, surgical masks and N95 respirators (Fig. 1) have gained significant media attention, both for their use as personal protective equipment (PPE) and their limited supply. While the Centers for Disease Control and Prevention (CDC) does not recommend that the public wear N95 respirators, it is recommended that you wear a surgical mask if you are sick or are caring for someone who is sick. That means many people may be putting on a surgical mask for the first time, and it is important that the correct side of the mask is facing out.
 Fig. 1 – Representative images of an N95 respirator (left, credit: Brian McGowan) and surgical masks (right, credit: Noah Matteo).
Fig. 1 – Representative images of an N95 respirator (left, credit: Brian McGowan) and surgical masks (right, credit: Noah Matteo).
Most surgical masks have a white side and a colored side as shown in Fig. 1, and you always want the colored side facing outward. This is because the two sides have opposite functions: the colored side is designed to repel liquid, while the non-colored side is designed to wick moisture away from your face. But what is it about these materials that makes water repel or stick?
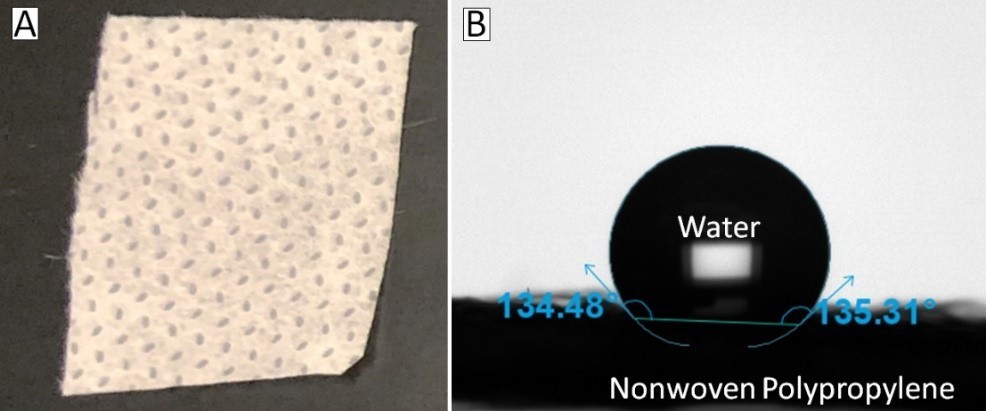
Fig. 2 – A piece of the nonwoven polypropylene outer layer of a face mask (A), and a water drop on the same sample used to measure the contact angle with a Theta Flex optical tensiometer (B).
The outside layer of the mask is typically a nonwoven polypropylene sheet. Polypropylene is a naturally hydrophobic polymer, meaning it “fears water” and causes water to bead up on its surface. For example, we took a piece of this outer layer (Fig. 2A) and placed a 3 µl drop of water on it using an Attension Theta Flex optical tensiometer (Fig. 2B). The angle between the polypropylene and the drop’s tangent is the contact angle, which in this case is 134.5° on the left and 135.3° on the right (Fig. 2B). When this angle is >90° the material is considered hydrophobic, and the closer it is to 180° the easier the drop will roll off, much like a drop of water on a windshield. Most importantly, this means any viruses and bacteria hitching a ride on airborne droplets will not stick to your mask!
The reason for this behavior has to do with interactions at the molecular scale. The attractive forces between molecules in the liquid drop and molecules in the polypropylene dictate how well the liquid will adhere. Water, being a very polar liquid, has a weak attraction to the non-polar polypropylene, and prefers to minimize its contact with the polymer. As a result, the water beads up, minimizes the surface area that it shares with the polypropylene, and will even roll off if given the chance.
The inside material of the mask is just the opposite. It is typically a fibrous nonwoven layer made of a hydrophilic polymer composite, such as polyethylene terephthalate and polyethylene. In this case, the attractive forces between the liquid and the inner mask material are stronger and the two materials prefer to share more surface area with each other, causing the liquid to wick into the material. Any germs you may have get stuck in the mask, limiting their ability to spread to nearby people.

Fig. 3 – The Attension Theta Flex optical tensiometer (Biolin Scientific).
Wettability is crucial in the design of surgical masks, but it is equally important for many applications including paints and coatings, personal care products, oil reservoirs, and semiconductors. Many of these industries routinely measure contact angles with an Attension Theta Flex optical tensiometer (Fig. 3) for product quality control and research and development.
We also offer these measurements and more as analytical services through our ISO 9001:2015 certified sister company Nanoscience Analytical.
