The need for implantable devices and biocompatible materials is driven by the demand for advanced medical solutions that can repair, replace, or support damaged tissues while minimizing the risk of rejection and complications. These technologies are essential for improving patient outcomes in a wide range of surgical and therapeutic procedures. Electrospinning, a versatile technique for producing ultra-fine fibers, is a powerful tool in the design, fabrication, and modification of implantable medical devices. By creating fibers at the nanoscale, electrospinning enables production of materials with high surface area, porosity, and unique mechanical properties, allowing mimicry of natural tissues. Electrospun materials can not only improve the integration of implants with surrounding tissue, but also allow for controlled drug delivery and targeted healing, making electrospinning a key technology in advancing the effectiveness and safety of implantable medical devices.

Electrospun Coatings on Prefabricated Medical Implants
Improving Biocompatibility and Drug Delivery
Electrospinning has shown amazing progress in applying biocompatible materials for stent coatings and other prefabricated medical devices. For instance, electrospun polymer coatings on stents have shown promise in improving biocompatibility and drug delivery. Polymers like poly(ε-caprolactone) (PCL) and polylactic acid (PLA) are commonly used for these coatings, offering controlled degradation and excellent mechanical support while reducing inflammation and thrombosis risks. Materials like polyurethane (PU) and PCL are also commonly electrospun to create nanofibrous coatings that enhance the biocompatibility of stents. These coatings not only improve the mechanical properties of the stents but also enable the localized delivery of drugs to prevent restenosis. A few commercially available examples of this technology are seen in the Caladrix® Technology (Electrospinning Company) 1 and BiowebTM PTFE/PU composite membrane (Zeus) 2. These electrospun coatings allow for sutureless robust coating adhesion, low profile, inclusion of bioactive molecules, and many more advantages to be applied to prefabricated devices. Images in Figure 1 show highlights of the stent coating process, along with uncoated versus coated stent samples.


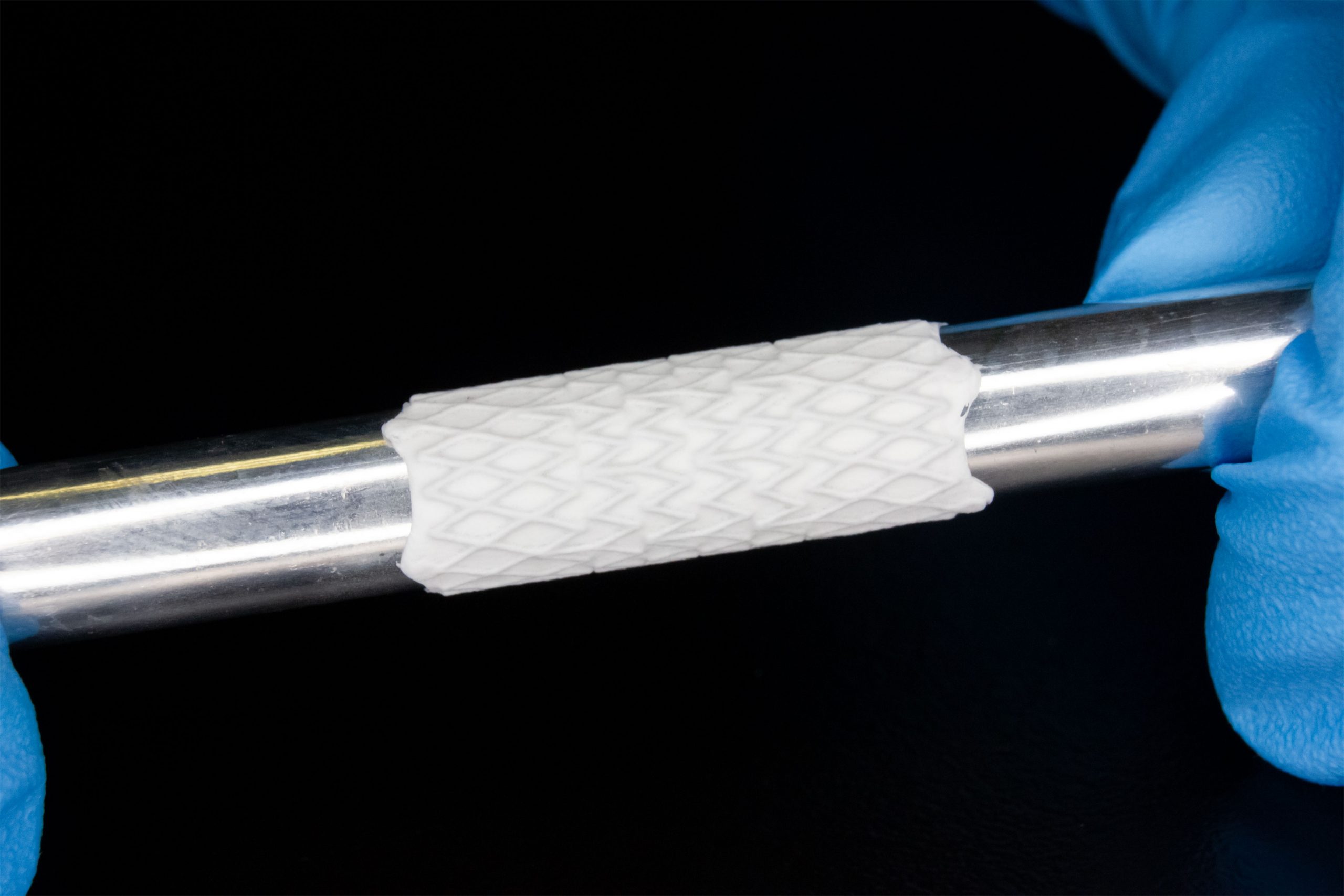
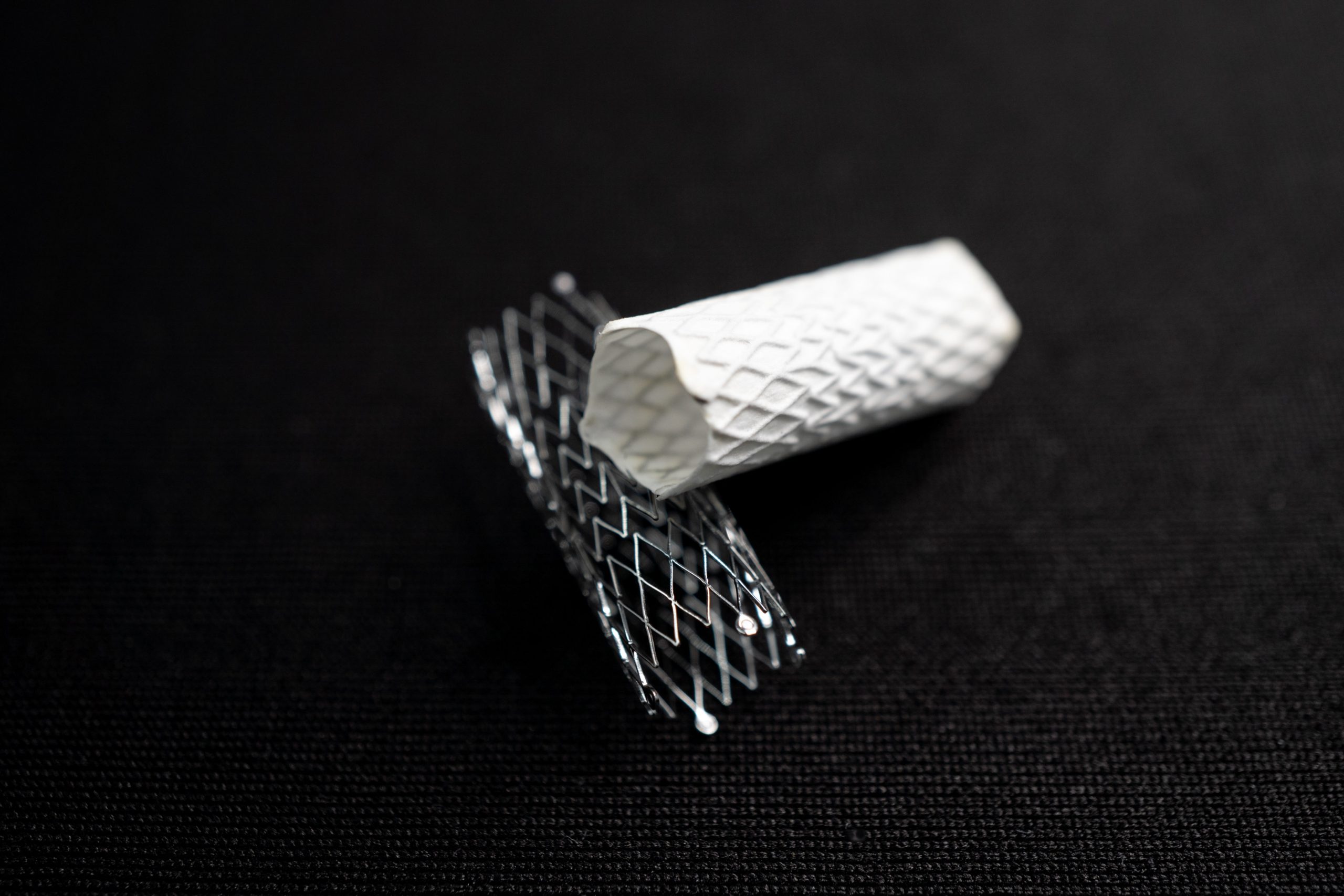
Improving Tissue Integration and Mechanical Properties
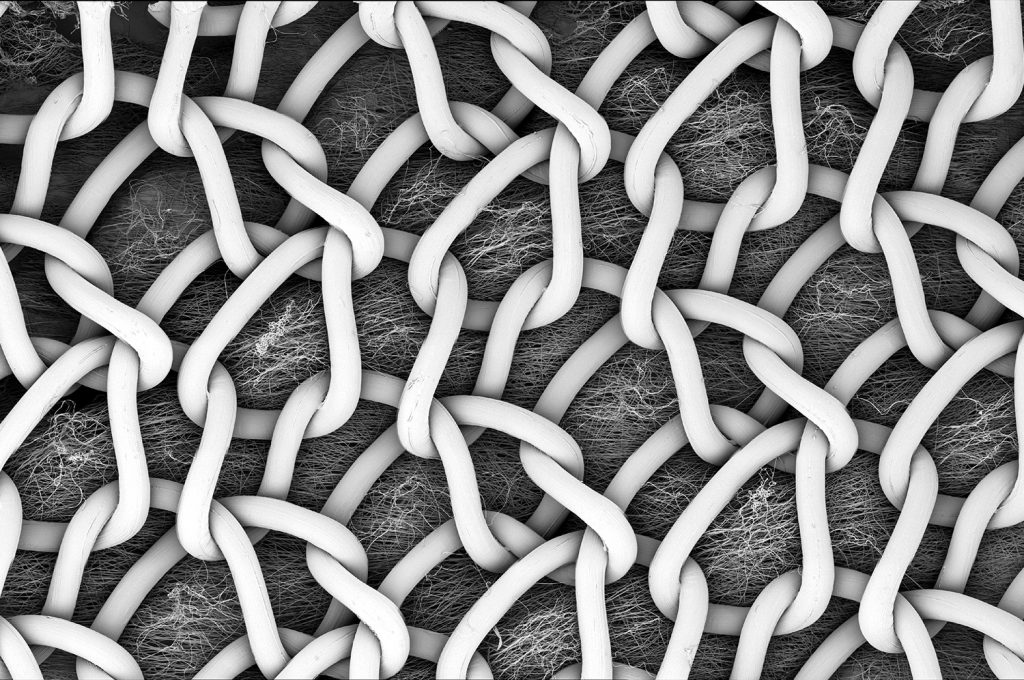
Similarly, hernia meshes with electrospun coatings are gaining attention due to their flexibility, strength, and ability to integrate seamlessly with surrounding tissues. (Figure 2) These meshes, often composed of materials like polypropylene (PP) and polyhydroxybutyrate (PHB), are then coated with electrospun biocompatible materials like poly(lactic-co-glycolic acid) (PLGA), to offer improved patient outcomes by minimizing immune response and maintaining mechanical support of the surrounding tissue while promoting tissue regeneration.3
Fully Electrospun Medical Implants
Improving Tissue Integration and Mechanical Properties
Beyond coatings, fully electrospun implants are pushing the boundaries of medical innovation. These implants are made from biocompatible materials that allow resorption into the native tissue, promotion of cellular ingrowth, and robust mechanical properties. For example, the commercially available Atreon Orthopedics ROTIUM® Bioresorbable Wick is a poly-glycolic acid (PGA) and poly-lactide co-caprolactone A (PLCL) electrospun medical implant designed for use in rotator cuff surgery (Figure 3).
Its electrospun microfibrous structure aids in the healing and repair of the rotator cuff by creating a bioresorbable scaffold that promotes “scarless healing with strong tissue integration and remodeling of a functional enthesis.” 4 By enhancing the body’s ability to repair the damaged tendon, it aims to improve the overall outcomes of rotator cuff surgeries, reducing recovery time, increasing the likelihood of a successful repair, and reducing reinjury rates.
Acera Surgical’s Cerafix® Dural Repair material is another excellent commercially available example of an electrospun biocompatible material implant. Cerafix® is an electrospun bioresorbable scaffold designed specifically for dural repair and soft tissue reinforcement. This implant is made from a blend of synthetic polymers that gradually resorb within the body, providing temporary support while promoting tissue regeneration. The material’s high porosity and strength make it ideal for the necessary quick tissue ingrowth and mechanical support required in dural applications where prevention of CSF leakage is critical.5 6
Improving Drug Delivery and Mechanical Properties
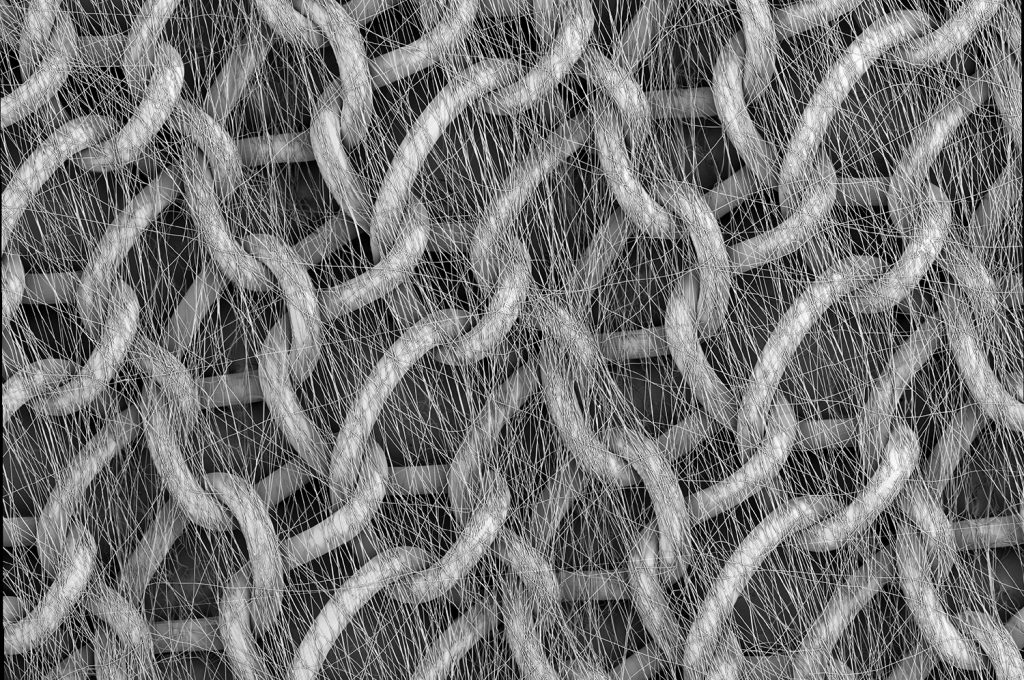
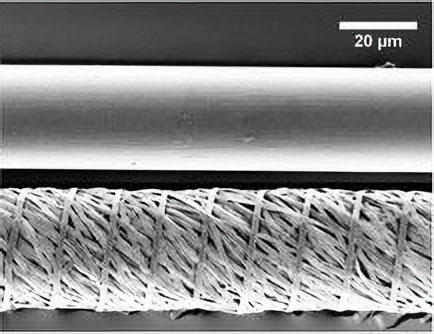
Electrospinning of multifilament sutures has also emerged as a promising application of biocompatible electrospun materials. Electrospinning these nonwoven multifilaments allows for small-scale retention, enhanced mechanical properties, and more effective drug elution compared to traditional sutures. For example, researchers at Johns Hopkins University (JHU) School of Medicine found that extremely thin sutures intended for ophthalmic surgeries can be formed by electrospinning drug-loaded PCL nanofibers “twisted” into a single multifilament (Figure 4). This multifilament suture then provided antibiotic elution directly at the suture site decreasing infection rates while maintaining structural integrity necessary for the microsurgical ocular procedures.7
Conclusion
Electrospinning is greatly progressing the field of implantable devices and biocompatible materials. Whether through enhancing prefabricated implants with advanced coatings or developing entirely new types of implants, electrospinning is paving the way for more effective, safer, and patient-friendly medical devices. As research continues, the potential for electrospinning in medical applications will only expand, promising a future where implants are more tailored to individual needs and outcomes.
References
- https://www.electrospinning.co.uk/stent-cover-technology/ ↩︎
- https://www.zeusinc.com/products/biomaterials/bioweb-composites ↩︎
- https://textile.webhost.uoradea.ro/Annals/Vol%2022%20no%202-2021/Textile/Art%20480%20pag%2039-42.pdf ↩︎
- https://www.atreonortho.com/Products/ ↩︎
- https://acera-surgical.com/cerafix/ ↩︎
- MacEwan, Matthew R., et al. “Novel nanofabricated dura substitute effectively repairs dural defects independent of defect size in a canine duraplasty model.” Interdisciplinary Neurosurgery, vol. 14, Dec. 2018, pp. 150–155, https://doi.org/10.1016/j.inat.2018.08.006. ↩︎
- Parikh, Kunal S., et al. “Ultra‐thin, high strength, antibiotic‐eluting sutures for prevention of ophthalmic infection.” Bioengineering & Translational Medicine, vol. 6, no. 2, 9 Dec. 2020, https://doi.org/10.1002/btm2.10204. ↩︎