A potentiostat is an electrochemical instrument used to control the potential difference between a working electrode and a reference electrode while measuring the resulting current. Potentiostats play a pivotal role in electrochemical research and analysis, providing valuable insights into the behavior of materials and systems under varying electrochemical conditions. The accuracy and reliability of these measurements depend significantly on the resolution of the potentiostat.
Resolution
The resolution of a potentiostat defines the smallest change in voltage or current that can be reliably detected and quantified. Higher resolution means finer measurement granularity, enabling researchers to capture subtle electrochemical phenomena and extract more detailed information from their experiments.
Voltage resolution is the smallest change in potential that the instrument can detect and control. It is typically expressed in millivolts (mV) or microvolts (µV). High voltage resolution allows researchers to measure subtle changes in electrochemical behavior which is particularly important in battery testing.
Current resolution represents the smallest detectable change in current that the potentiostat can measure accurately. It is dependent on the lowest full-scale current range that a potentiostat possesses. It is usually expressed in nanoamperes (nA) or picoamperes (pA). The precise current resolution is essential for studying electrochemical processes with low current densities, such as the detection of trace analytes, impedance spectroscopy, and electrochemical deposition.
Accuracy and Precision
Accuracy and precision are often used interchangeably, but in scientific terms have different meanings. Accuracy pertains to the closeness of the results to the “correct” value. Precision refers to the closeness of the individual data points to each other. It’s possible to have accurate results that are not very precise and conversely, precise results that are not accurate. Improved resolution is essential among other factors in obtaining the most accurate and precise results.
How is Resolution Defined?
In modern potentiostats, the resolution is defined in terms of the “number of bits in an ADC”. This defines the resolution of the analog-to-digital converter used in the potentiostat. But how does this translate into the resolution of measurement?
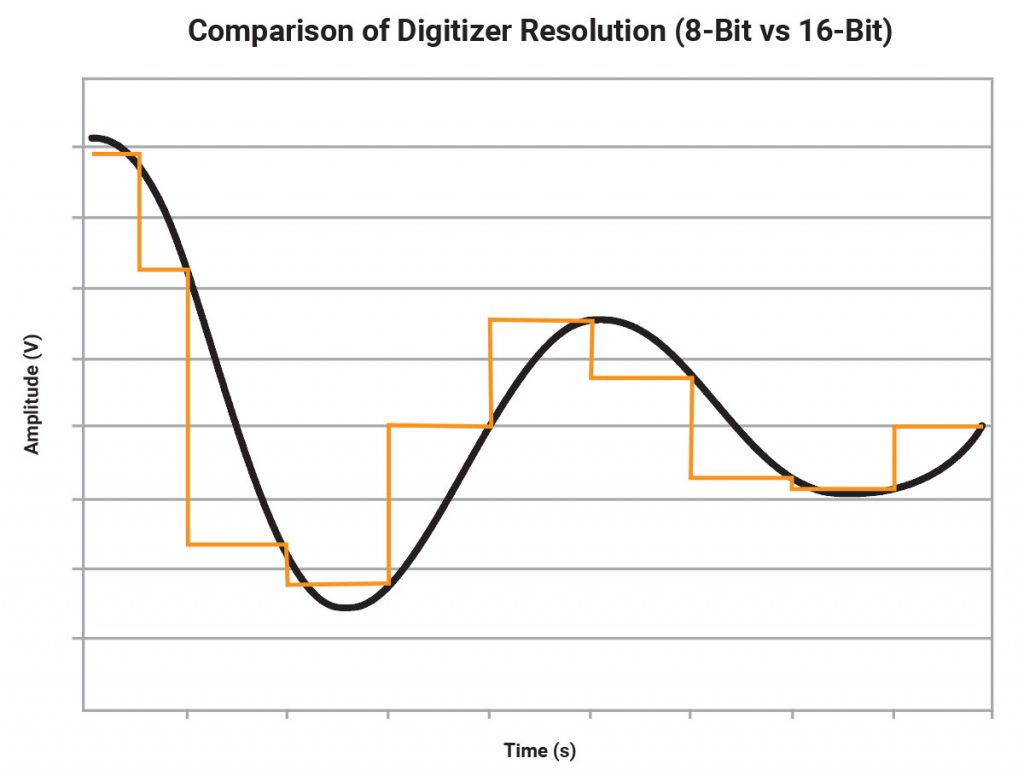
The applied and measured voltage and current signals in an electrochemical system are continuous. However, to enable digital computers to interact with these signals, they are digitized using digital-to-analog and analog-to-digital converters. To digitize a continuous signal, the signal is sampled and quantized resulting in loss of some segments of the continuous signal. To illustrate this concept, consider an analog signal of a voltage (Fig 1) and the corresponding signals when passed through an ideal 8-bit and ideal 16-bit ADC. An 8-bit ADC divides the range of the signal into 256 discrete levels. So, even in ideal situations, an 8-bit ADC cannot resolve voltage differences of less than 3.92 mV in a 1V range. However, a 16-bit ADC can ideally resolve voltage differences as small as 15 µV.
The digital signal will be equivalent to the continuous signal plus a quantized error. Since the quantized error is a function of the number of bits, the equivalence of the digitized signal to the continuous signal is determined by the resolution of the ADC. Higher precision of the measurements is achievable with higher ADC resolution.
If the full-scale range of the signal is relatively small, then a lower-resolution ADC can be used to measure small signals. However, in many cases, the signals contain both large and small signal components, and therefore, a high-resolution ADC is required to measure the full range of signals.
Why is Resolution Important in Potentiostats and Electrochemistry?
Resolution is important in many experiment types that the electrochemist uses. While potentiostats in general have many consecutive current ranges, many experiments have to be recorded on a fixed current range either because of the speed of the experiment or because of large, fixed background currents or voltages. These include cyclic voltammetry, pulse techniques, electrochemical impedance spectroscopy, fast current and voltage steps, and battery tests.
For example, in cyclic voltammetry, at scan rates above 20 mV/s, automatic current ranging can add unwanted spikes or glitches in the current measurement as the switching of the current range has a finite time. In addition, if you wish to compensate for the iR voltage drop due to the solution resistance this can only be successfully achieved using a fixed current range. The use of fixed current ranges in these cases is required and can make it difficult to resolve small current peaks at the same time as larger ones.
Electrochemical impedance spectroscopy experiments typically use small AC perturbations to interrogate the measured system. Small signal responses require a high degree of resolution to make the most accurate measurements as a large background voltage or current may be present with the sine wave signal.
Conclusion
Resolution plays an important role in obtaining accurate and precise results in electrochemical and battery testing experiments. High resolution enables the study of a wide range of electrochemical phenomena with confidence, leading to a better understanding of fundamental processes and the development of new electrochemical technologies and applications.