Electrospinning & Electrospraying System
Fluidnatek® LE-50 Prosterile
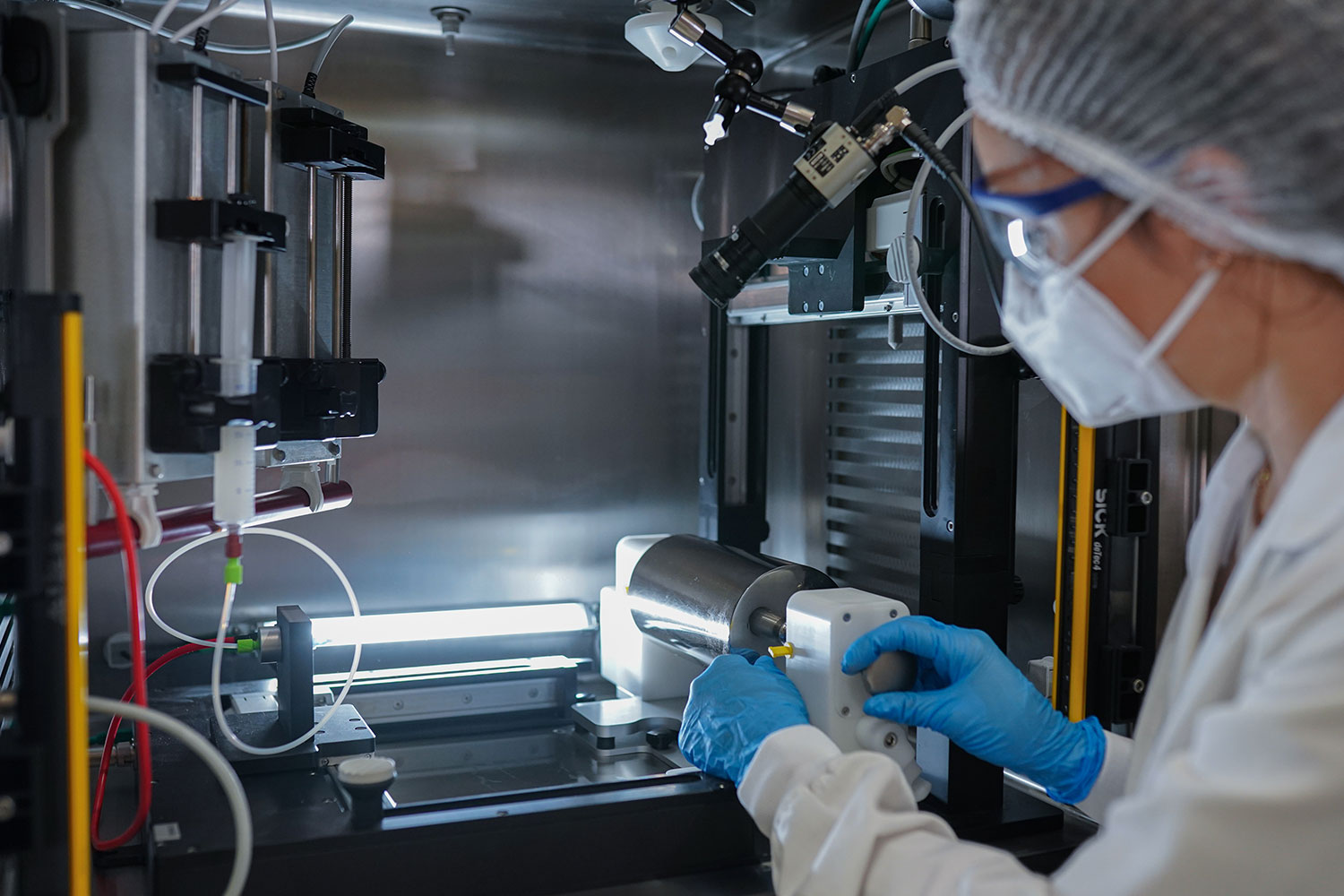
The Fluidnatek LE-50 ProSterile is the ideal solution for biomedical companies requiring aseptic electrospun sample development for medical products under ISO-5/Class 100 cleanliness, cGMP, and ISO-13485 certification standards. It is the instrument of choice for in-line aseptic electrospun bioprocesses, and medical device manufacturing of sterile products. This system has elevated the electrospinning technique into a new era of ultra-clean medical device processing and personalized medicine.
Maintain Sterility
Multiple methods of sterilization ensure a sterile product throughout processing.
ISO-5 / Class 100 Cleanliness
Ultra-clean medical device processing for personalized medicine.
Exclusive Environmental Control Unit
Powerful system for stable and consistent conditions during sample development.
Process Validation
Qualification documents (FAT, SAT, IQ, OQ) provided for process validation like ISO, cGMP, GLP, GAMP, and FDA.
Talk to an Instrumentation Specialist Today!
The Only Electrospinning System Equipped for Sterile Development of Medical Products
The LE-50 ProSterile is the first electrospinning system to offer a fully sterile process environment, enabled by its welded 316L stainless-steel interior and exterior (no crevices or gaps improves cleanliness), HEPA filtration to meet ISO-5/Class 100 cleanliness standards, and a chamber sterilization system. Glove port access allows sample handling and packaging under sterile conditions, and the vacuum antechamber removes possible traces of solvents while maintaining an exceptional standard of sterility and cleanliness. An environmental control unit (ECU) enables precision control of temperature (20 – 45 °C) and relative humidity (10 – 80%) to maintain batch to batch reproducibility for perfect nanofibers, every time.
Application Areas:
Fluidnatek LE-50 Prosterile
Product Features
Aseptic Processing
Designed with cleanrooms and personalized medicine in mind, the LE-50 ProSterile adheres to ISO-5/Class 100 standards and enables aseptic fabrication of health care products. Using a HEPA-equipped ECU and various sterilization methods, the ProSterile maintains a clean and sterile environment inside of the chamber.
The LE-50 ProSterile is constructed entirely from 316L stainless steel without gaps or crevices, allowing for complete sterilization of all internal surfaces and improving cleanliness. It is also equipped with glove ports on the front of the unit for changing the configuration of the emitter and collectors or manipulating samples inside the vacuum antechamber. A vacuum sealed box is built into the unit that prevents contamination while transferring products out of the processing chamber. It is also used to remove possible traces of solvents under ISO-5/Class 100 conditions.

Full Environmental Control with HEPA filtration
The Fluidnatek Environmental Control Units provide exceptional control over the temperature, humidity, and air flow in the chamber of the electrospinning system during processing. The LE-50 ProSterile has a built-in ECU that uses HEPA filters to actively filter conditioned air that is pumped into the processing chamber and removes solvent vapors during active electrospinning or electrospraying processes. Conditioned air helps to boost the consistency of electrospun materials and enables batch-to-batch reproducibility of defect-free samples.
Suitable for GMP and ISO Processing
Fluidnatek’s instruments, including the LE-50 ProSterile, are designed with features that facilitate compliance with various regulatory standards in industries like medical devices and pharmaceuticals. Supporting current Good Manufacturing Practices (cGMP), the LE-50 ProSterile can be integrated and process validated into environments requiring ISO-13485 certification, which is crucial for medical device manufacturing. Instrument validation services are available, including IQ/OQ documentation by expert technicians. Process development and qualification (PQ) can be completed as well. Optional validation services can also be completed in compliance with good lab practices (GLP), good automation manufacturing practice (GAMP), and the US Food and Drug Administration (FDA).
Batch-to-Batch Reproducibility
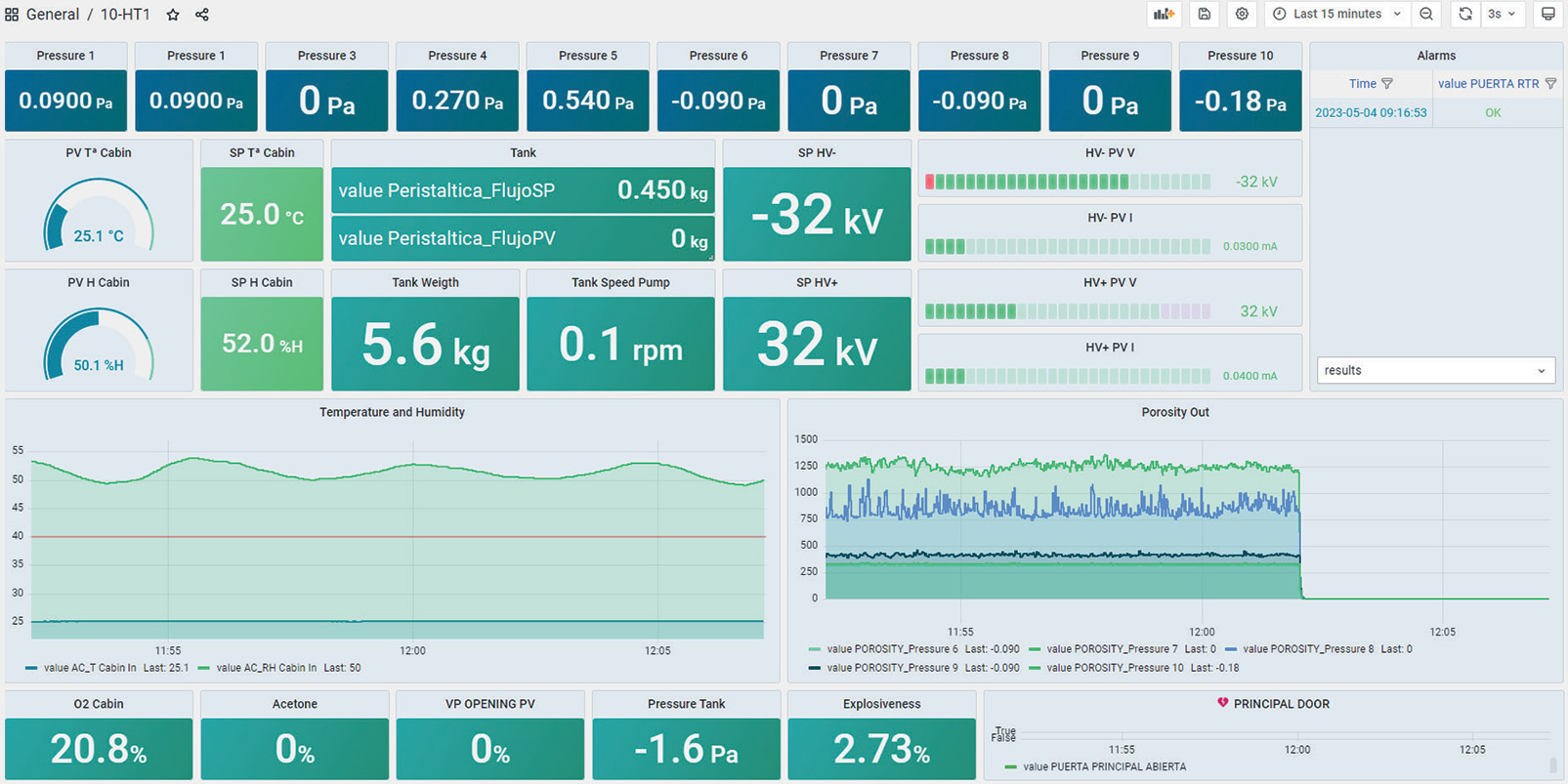
Consistency must be maintained when fabricating medical and pharmaceutical products to ensure standards and specifications are met. The optional High-definition Process Data Hub enables monitoring of all processing parameters and stages throughout the electrospinning process, along with internal signals of the machine. Some valuable datapoints that are captured continuously include temperature, humidity, voltage, flow rate and rotational speed, and needle translation. The system also creates an audit trail of actions taken on the instrument. These audit details can be exported and reviewed including: what buttons were pressed, if the door was opened or closed, and if any alarms set by the user have been triggered. This enables the advanced sensors to monitor parameters and for the user to evaluate how a batch has varied if the samples fluctuate post-development.
Advanced Sample Development
Multiple configurable and automated electrospinning emitter stages with independent high voltages and solution supplies allow for dual spinning (co-spinning) of multiple solutions simultaneously for complex sample development, such as: multi-layered combination or bipolar membranes. The LE-50 ProSterile accommodates the production of fiber-fiber, fiber-particle, particle-particle and even fiber-live microorganisms (or cells) samples in various material combinations and form factors.
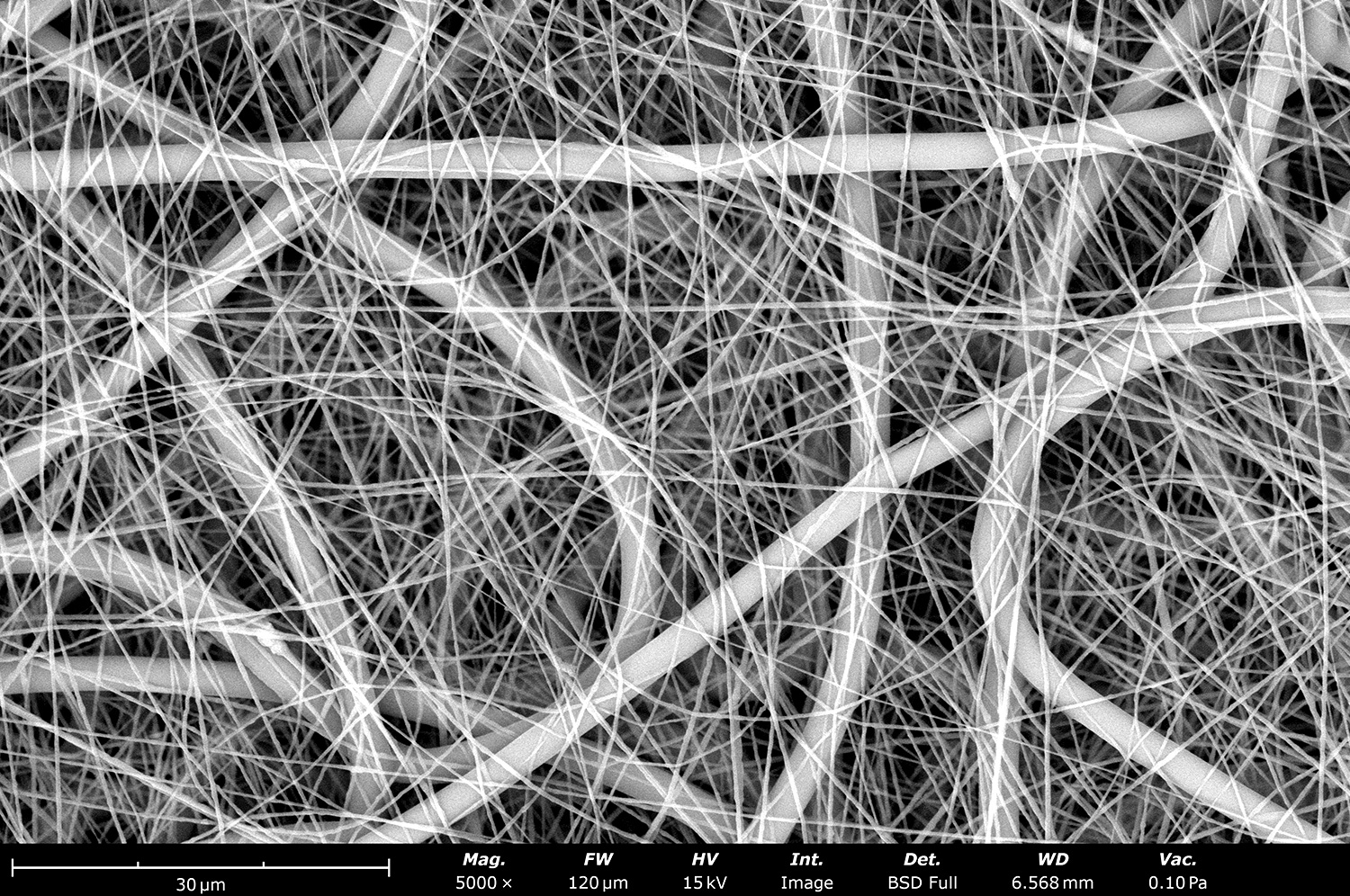
Versatility
The LE-50 ProSterile offers versatility through a wide range of compatible materials, solvents, and electrospinning configurations (over 30+ accessories are available). Researchers have the flexibility to experiment with various materials (polymers, ceramics, metals, additives or their blends), emitters (single-phase, coaxial, triaxial, multi-needle, needle-less, among others), spinning orientations (vertical, angled, horizontal), and collectors (flat plate, drum, mandrel, disk, liquid reservoirs) to fabricate nanofibers and particles with different properties for numerous applications all in a single integrated electrospinning unit.
Safety Features
Numerous safety features are built into the LE-50 ProSterile system, including a chamber door interlock, password-protected operator access, regulated airflow for solvent vapor removal, emergency shutoff, and arcing detection. Extensive user training for instrument operators is available as well, to ensure safe use of the equipment for new users or as refresher training for current users.
Support & Service
Top-tier comprehensive US-based support and service for the LE-50 ProSterile and accessories, including installation assistance, training, maintenance, and technical support are available to help users maximize the performance and reliability of the equipment.
Fluidnatek LE-50 Prosterile
Product Accessories
High Definition Process Data Hub
High Voltage Options
With multiple independent high voltages available in the unit, bias can be applied to additional emitters and/or collector. Additionally, voltages can be upgraded to bipolar capacities of -30kV to +30kV (emitter) or -10kV to 10kV (collector). Multiple adjustable voltages and a secondary stage in tandem provide configuration flexibility, such as co-spinning or dual spinning/spraying, emitter/collector voltage inversion for specific orientation, or preferential sample deposition/morphology.
Multiple Syringe Pumps
The ProSterile can accommodate up to 3 single syringe pumps within the electrospinning chamber. Implementing 2 or more syringe pumps opens the door to multi-solution processing for coaxial, triaxial, or co-spinning (AKA dual spinning) sample formation. Simultaneous use of multiple solutions enables the creation of composite or layered fibers with diverse properties for advanced applications. This setup also increases productivity by enabling higher throughput and flexibility in controlling fiber characteristics, such as morphology and composition, for producing more complex and multifunctional materials.
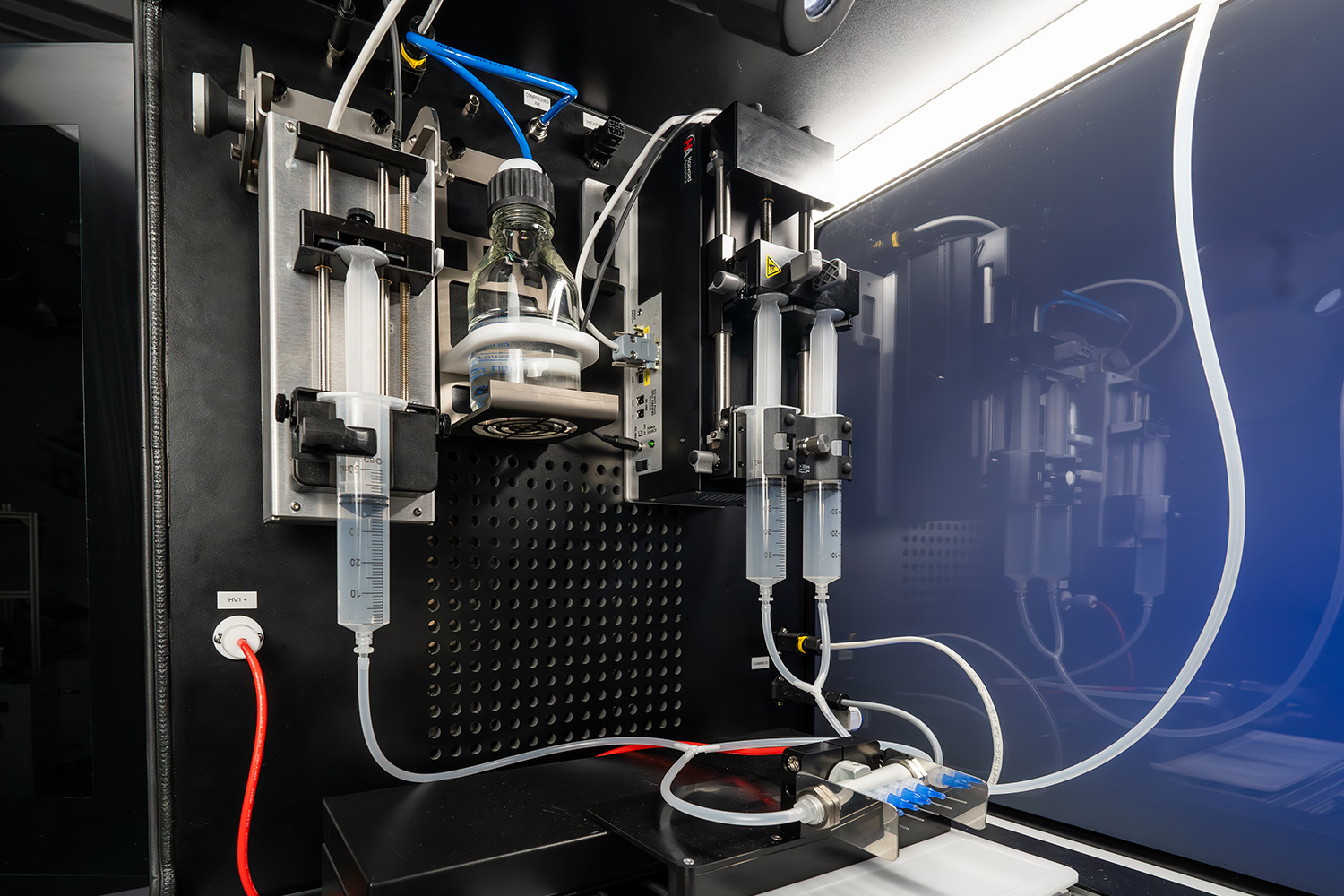
Needle Translation
The ProSterile equipped with improves sample homogeneity and thickness with needle translation. This helps produce more uniform fibers by distributing the electrospun solution more evenly across the collector, reducing fiber defects and ensuring consistent fiber diameter. Additionally, it enhances productivity by increasing the deposition area, allowing for the fabrication of larger, more uniform samples efficiently. Translating the needle allows users to coat particular areas on a medical device (ex. heart valve or stent) in a desired area without wasting expensive materials.
Adjustable Emitter Orientation
ProSterile compatible emitters can be positioned vertically and horizontally on independent spinning stages or angled (using an articulating flexible arm). Vertical orientation for top-down spinning or spraying onto collectors or water bath providing options for improved solution processing or particle collection. Horizontal and angled positioning prevent solution dripping onto collectors, easily allowing solution optimization or low viscosity solutions to be processed without defect to the final sample.
Taylor Cone Visualization System
The Taylor cone visualization system uses a high-quality camera to focus directly on the emitter needle tip for a close view of Taylor cone formation and solution processing. Taylor cone formation is indicative of the electrospinning quality and is therefore crucial for monitoring during process optimization. By observing the cone shape and stability in real-time, operators can quickly adjust parameters such as voltage or flow rate to ensure consistent fiber production and reduce defects. This leads to improved control over fiber diameter, morphology, and quality, resulting in a more efficient and reliable electrospinning process.

Emitter Variety
The LE-50 ProSterile accommodates both needle-based (single phase, coaxial, triaxial, multi-needle, gas-assisted) and needle-less (slit injector) emitter forms all in a single electrospinning unit. This extensive array of emitters means and equally extensive array of fibers and particles morphologies are possible, including core-shell, core-shell-shell, hollow, textured, porous, and many more. Gas-assisted and solvent-gas jacket configurations provide assistance in spinning difficult or low boiling point solutions. Additionally, multi-needle and slit injector (needle-less) options can increase overall throughput and sample productivity.
Collector Variety
In addition to a standard flat plate configuration for randomly oriented fibers, the LE-50 ProSterile can be equipped with a rotating drum, rotating mandrel, or rotating disk collector for various types of sample deposition. These collectors provide a controlled, dynamic surface for fiber deposition, leading to the production of aligned or oriented fibers, which can enhance mechanical properties such as tensile strength. Rotating collectors can improve uniformity and consistency in fiber distribution, and when paired with needle translation the continuous motion helps to avoid buildup in any one area, facilitating the production of non-woven mats with consistent thickness and density over larger areas for increased productivity.
Instrument Certifiability
The high-quality, professional nature of the Fluidnatek line provides the basis for GMP, GLP, GAMP, FDA, and/or ISO-13485 certifiable electrospinning machines. This certification enhances product reliability, consistency, and safety, making electrospun materials suitable for medical, pharmaceutical, and other regulated industries. It also builds trust with clients and stakeholders, demonstrating commitment to compliance and quality control, setting the machine apart from competitors and opening doors to broader market access and potential collaborations.
Fluidnatek LE-50 Prosterile
Electrospinning & Electrospraying Applications
Tissue Engineering
Electrospun nanofibers are used to create scaffolds for tissue engineering applications. These scaffolds mimic the extracellular matrix, providing a supportive environment for cell growth and tissue regeneration. They are prevalent in wound healing, bone regeneration, nerve regeneration, and more.
Bio Textiles
BioTextiles are fibrous materials used in healthcare and biomedical applications. Electrospinning offers a highly customizable method of fabricating biotextiles, allowing careful tuning of mechanical, chemical, and biological properties of the nanofibrous material. Electrospun biotextiles are typically biocompatible and can be fabricated from polymers or organic materials that are biodegradable or bioresorbable. Electrospun yarns can be loaded with active pharmaceutical ingredients (APIs) to create drug eluting sutures,
Medical Devices
Whether as coatings for prefabricated structures, active ingredient additives, or total device fabrication, medical devices implement electrospun nanofibers and particles for enhanced therapeutic effectiveness. These fibers and particles can allow for improved performance in mechanical properties, biological microenvironment control, cellular integration, and many more application-specific advantages.
Drug Delivery
Electrospun fibers and/or particles can be loaded with drugs or therapeutic agents and used as drug delivery systems. Precisely controlled electrospinning parameters allow the high surface area-to-volume ratio and tunable properties of nanomaterials, providing controlled release of drugs, making them suitable for targeted and sustained drug delivery.
Fluidnatek LE-50 Prosterile
Product Knowledgebase
Webinar
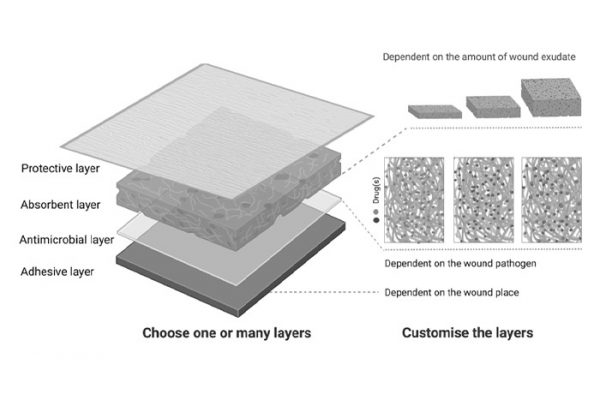
Innovative Drug Delivery Systems for Wound Healing Using Electrospinning
Chronic wounds and wound infections are a major problem for the society and novel treatmen…
Blog
Fabricating Complex Geometries with Electrospinning
Electrospinning has emerged as a transformative technique for fabricating fibrous material…
White Paper
Controlling the Orientation of Electrospun Nanofibers for Tissue Engineering
Electrospinning is a versatile and promising technique for fabricating nanofibrous scaffol…