What is Electrochemistry?
Electrochemistry is:
- The science of electron transfer across a solution-electrode interface.
- The study of chemical reactions involving the transfer of electrons.
- It is the study of chemical reactions where electricity is the driving force
There are two main branches in electrochemistry:
- Chemical reactions that generate electricity
- Chemical reactions that are driven by electricity
Electrochemistry has a wide range of applications, from integrity of coatings on metal structures and biomedical sensors at the low current end and electric vehicle batteries and fuel cell power generators at the high current end. Electrochemistry has applications both in fundamental research and in production environments.
Redox Reactions
Redox reactions or Reduction-Oxidation reactions are electrochemical half-reactions that describe the electrochemical conversion of ions or molecules. The generic form of a reduction reaction is given as:
Ox + ne- → Red
In general, when a negative going potential is applied to an electrode, a reduction occurs. Conversely when a positive going potential is applied an oxidation occurs.
An example of a reaction that can be split into half-reactions is:
Zn + 2HCl → ZnCl2 + H2
Oxidation Half-Reaction: Zn → Zn2+ + 2e–
Reduction Half-Reaction: 2H+ + 2e– → H2
The spontaneous nature of this reaction is governed by the free energy associated with the equilibrium of each half-reaction. In electrochemistry, this is governed by the Nernst Equation. The Nernst equation allows for the calculation of the cell potential based on the standard electrode potential.
E |
= |
E0 - |
RT |
ln Q |
||||
nF |
||||||||
Where E0 is the standard electrode potential and Q is the reaction quotient. The reaction quotient is the ratio of the activities of the products divided by reactants. In many situations the activities can be replaced by the concentrations.
The electrode potential can be calculated for any equilibrium concentration of reactants and products.
Galvanic Cells
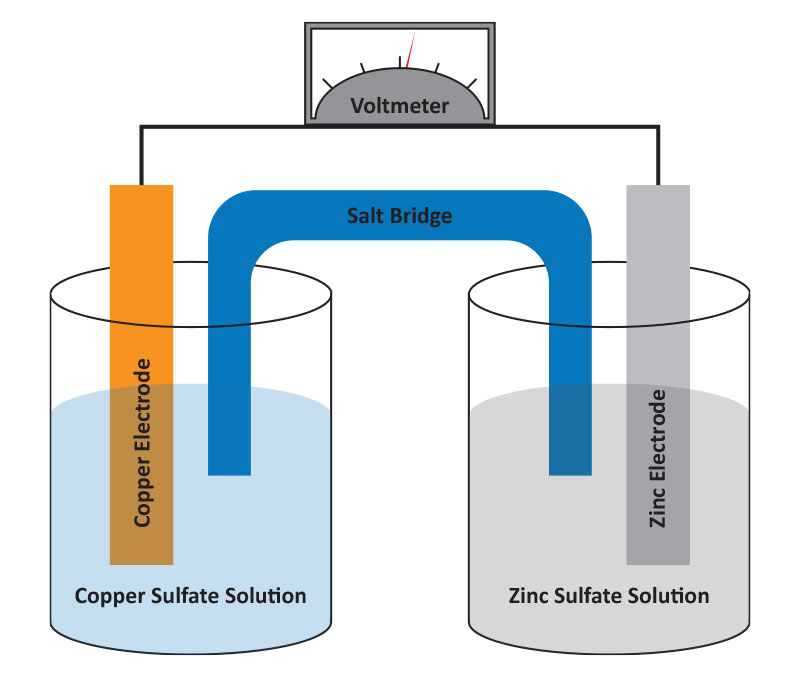
Galvanic cells are electrochemical cells created when a spontaneous reaction happens between two separated half-reactions, generating an electric current. A typical example is zinc and copper metal electrodes in separate beakers containing appropriate electrolyte salt solutions connected by a porous membrane. When an external wire is connected between the 2 metals, current flows because the zinc is dissolved and copper ions in solution are deposited as copper metal. Batteries are galvanic cells or a series of galvanic cells that contain all the reactants needed to produce electricity.
Electrolytic Cells
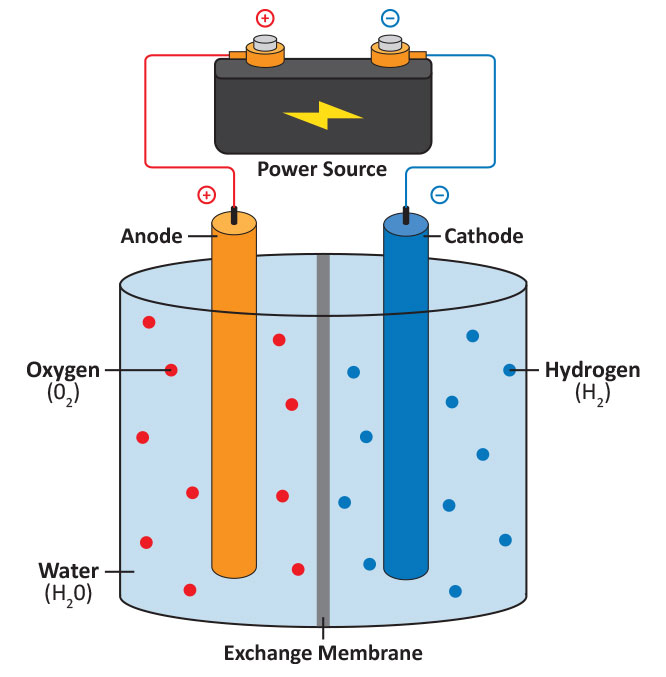
Electrolytic cells are electrochemical cells where electrochemical reactions are driven by the application of a voltage or current. An electrolytic cell consists of an anode, a cathode, and a container. An example is an electrolysis cell for the decomposition of water into its constituent components, hydrogen and oxygen.
Electrochemical Measurement Tools
Potentiostat
The essential tool for making electrochemical measurements is the potentiostat, a device for accurately measuring current and voltage. Typically, potentiostats can measure currents over a wide range, from amps to picoamps, and this accounts for the variety of applications. Some of these applications include battery testing, coatings evaluation, corrosion rate determination, and sensors.
Electrochemical Measurement Cell
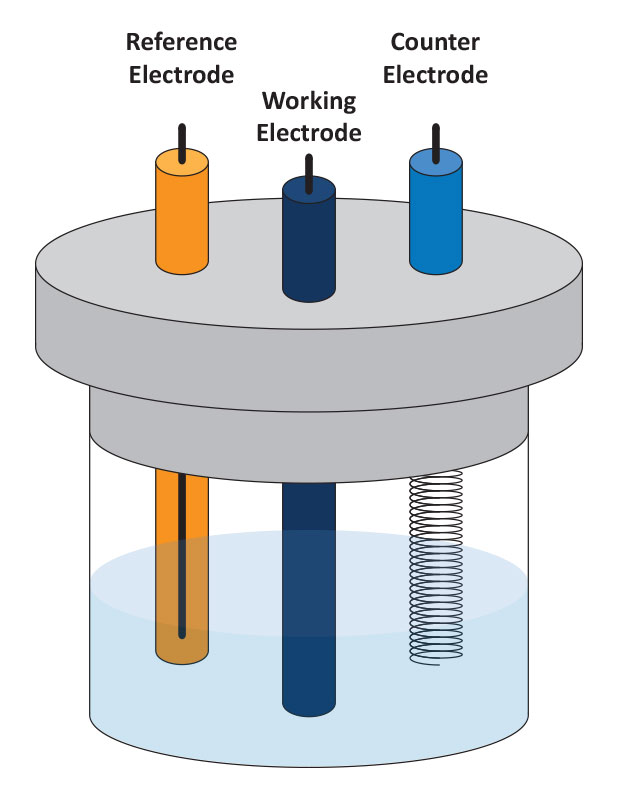
The measurement cell is an essential part of electrochemical experiments. The components of the cell are the working electrode, where the reactions of interest take place, the reference electrode, a standard half-cell to provide the voltage reference point, and the counter or auxiliary electrode that completes the circuit to allow current to pass through the cell. The cell can be as simple as a glass beaker with three electrodes suspended in an electrolyte solution. The solution in the cell can be a mixture of an electrolyte salt and the electroactive compound of interest. In some cases, these are one and the same. (Schematic of an electrochemical cell)
Components of the Electrochemical Cell
Working Electrode (WE)
The Working electrode is the electrode where the electrochemical reactions are studied. When studying solution electroactive species, the working electrode should be made of an inert material that does not undergo significant chemical or structural changes during the measurement. Examples of suitable electrodes are platinum, gold, or glassy carbon.
Reference Electrode (RE)
The Reference electrode is the electrode with a fixed, known potential, against which the applied potential is measured. The characteristics of the reference electrode are that it should be stable during the measurement and allow for the focus to be on changes at the working or sample electrode.
Counter (or Auxiliary) Electrode (CE)
The counter or auxiliary electrode completes the circuit and allows current to flow into the cell for the electrochemical reaction of interest. It should also be composed of an inert material with a larger area than the working electrode. A larger area is ideal, although not required, to minimize the voltage drop at the counter electrode. Examples of counter electrodes are coiled platinum wires or platinum gauzes.
Electrolyte Solution
The electrolyte solution used in experiments is an important component of the electrochemical system. The electrolyte solution consists of several ingredients, including the electrolyte salt and the solvent. The ideal properties of the electrolyte solution are chemical and electrochemical inertness in the proposed experiment, with good conductivity.
The modes of transport of the reactants in an electrochemical cell are diffusion, migration, and convection. To simplify the understanding of the electrochemical system, migration, and convection are undesirable and should be mitigated. Migration is mitigated by having a high concentration of electrolyte salt relative to the reactant, and convection is mitigated by controlling the experimental timescale as well as eliminating stirring and vibrations.
Physical electrochemistry
Physical electrochemistry is a branch of electrochemistry that focuses on the study of the fundamental physical principles underlying electrochemical processes. It deals with the relationship between electrical and chemical phenomena, providing insights into the mechanisms, kinetics, and thermodynamics of reactions occurring at the interfaces between conductive materials and electrolytes.
An understanding of the behavior of the electrode-electrolyte interface is fundamental as this is where charge transfer and mass transport interplay. This interplay governs electrochemical reactions, and it is crucial to elucidate the factors affecting the structure and composition of the interface to improve electrochemical processes. In particular, both the electrochemical kinetics and thermodynamics yield information about the speed and direction of electrochemical reactions through an understanding of the Butler-Volmer relationship, and the Nernst equation, respectively.
Physical electrochemistry has numerous practical applications, including battery and fuel cell development, electroplating, sensor technology, and electrochemical machining.