Get an early indication of potential incompatibilities and identify ways to mitigate.
In this webinar you will learn about QSense QCM-D technology and how it can be used to analyze adsorption of biologics and excipients to materials used in manufacturing, storage, and administration of biopharmaceuticals to provide an early indication of potential incompatibilities, and to identify ways to mitigate.
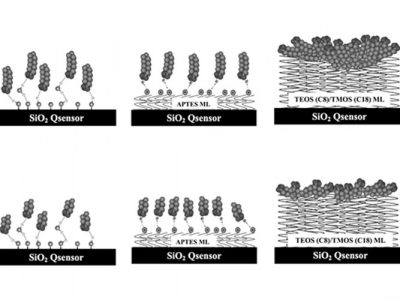
Dr. Erik Nilebäck, senior application scientist at Biolin Scientific, will introduce you to the QSense solution and take you through two case examples showing how QSense can be used to assess biopharmaceutical interaction in the context of pre-filled syringes. The case examples are then followed by a brief demonstration of the QSense Omni instrument and how a QCM-D measurement is run in practice.
In this webinar:
- Introduction to QSense solution
- Case example demonstrating how QSense QCM-D can be used to assess biopharmaceutical interaction
- Demonstration of QSense Omni
- Q&A