During the manufacturing and delivery process therapeutic proteins encounter several surfaces and consequently, face the risk of aggregation. There are several factors that can induce aggregation, such as temperature, shipping, storage, and physical stress. Aggregation can lead to loss of immune response or unwanted cross-reactivity and is typically remediated via addition of excipients to stabilize a formulation.
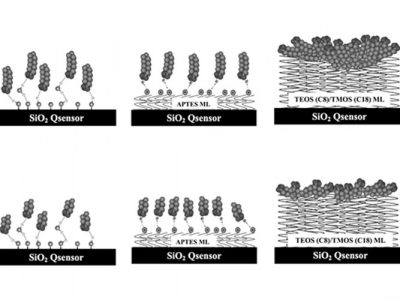
The Quartz Crystal Microbalance with Dissipation (QCM-D) technique can be used to evaluate how proteins interact with manufacturing vessels, processing materials such as filters, storage containers, or delivery devices. With this technique the amount of protein that interacts with a surface can be quantified as well as the resulting structural (viscoelastic) properties. The presence or lack of excipients, and other formulation conditions such as concentration, pH or type of buffer can be evaluated to determine what effect they have on the adsorption characteristics.
In this webinar we will discuss the interactions of model proteins with various surfaces under different formulation conditions such as increasing protein concentration and presence of excipient (PS 80 and Poloxamer 188). Data from previously published results of therapeutic protein/surface interactions will also be discussed.