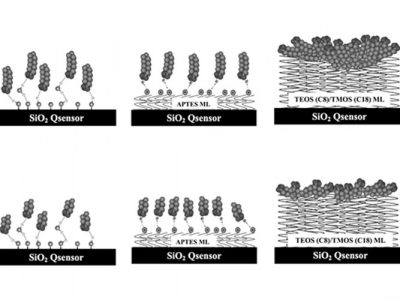
A biopharmaceutical drug encounters several surfaces during its lifecycle, from production to delivery. The stability and efficacy of these drugs can be significantly influenced by their interactions with various materials.
QSense QCM-D has emerged as a leading tool for examining these interactions, thanks to its exceptional mass sensitivity and a broad array of sensor surfaces, including metal, glass, and polymer options that simulate real-world environments.
The unique features of QSense latest model QSense Omni makes it ideal for Biopharma related research:
- High mass sensitivity < 0.3 ng/cm2 (enables quantifying antibody drug loss due to surface adsorption down to 0.1 ug/ml)
- Improved electronics decrease signal noise -> measure smaller binding events
- Small sample volume down to 90ml
- Quick analysis time: minutes to hours
- 5x faster fluid exchange -> sharper sample delivery
- Improved temperature stability (±0.003), and wide temperature range: 4 °C – 70 °C.
- Automated features minimize user-dependent data variation
- Improved workflows and intuitive new software make QCM-D easier to use.
In this demonstration, we’ll guide you through each step of a QCM-D experiment—from instrument setup to data analysis – including:
- Experimental Setup: Sensor mounting, sample preparation, QSense Omni configuration, and creating the experimental script in Omni software.
- Omni Software Features: Leveraging automated quality control, live script editing, and automated cleaning post-experiment.
- Real-time Data Monitoring: Observing antibody adsorption on four surfaces—Borosilicate glass, Stainless Steel, Polypropylene, and PVC.
- Data Analysis: Interpreting results to assess material compatibility and inform stability strategies.
Join us as we explore how QSense QCM-D can transform biopharmaceutical surface compatibility studies!