Redox-active polymers have promising applications in electronics and energy storage due to the polymer’s tunable conductivity and redox activity. For example, the conductivity of poly(3-hexylthiopene) (P3HT) is heavily dependent upon the doping level and the dopant type. This feature becomes especially important when considering P3HT or similar conjugated polymers for devices that require switching between electronic states (conductive vs insulating).
In another example, radical-containing polymers such as polyTEMPO can store energy through the transport of electrons and ions as the TEMPO groups reversibly oxidize and reduce.
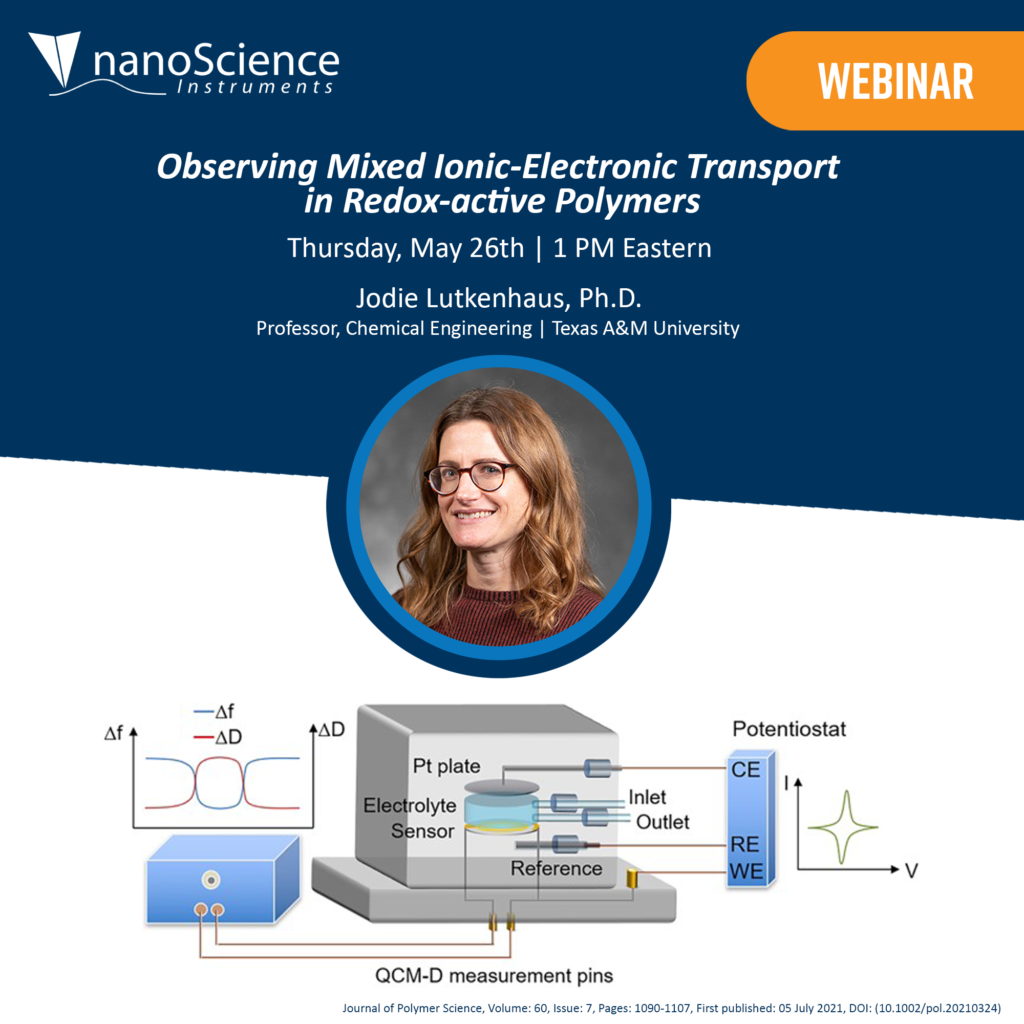
In this talk, the mechanisms of mixed ion-electron transport are examined using electrochemical quartz crystal microbalance with dissipation monitoring (EQCM-D). During cyclic voltammetry and galvanostatic charge-discharge experiments, the mass change of a redox-active polymer film is monitored in real time. Distinct mass transport regions are quantified as a function of doping level and potential, which are then correlated to changes with in situ conductance, spectroelectrochemical response, and energy storage. To identify the time scale at which the reaction transitions from kinetic to diffusion control, electrochemical impedance spectroscopy is coupled with EQCM-D.
This work gives valuable insight into the nature of mixed ion-electron transfer, including its time scale.