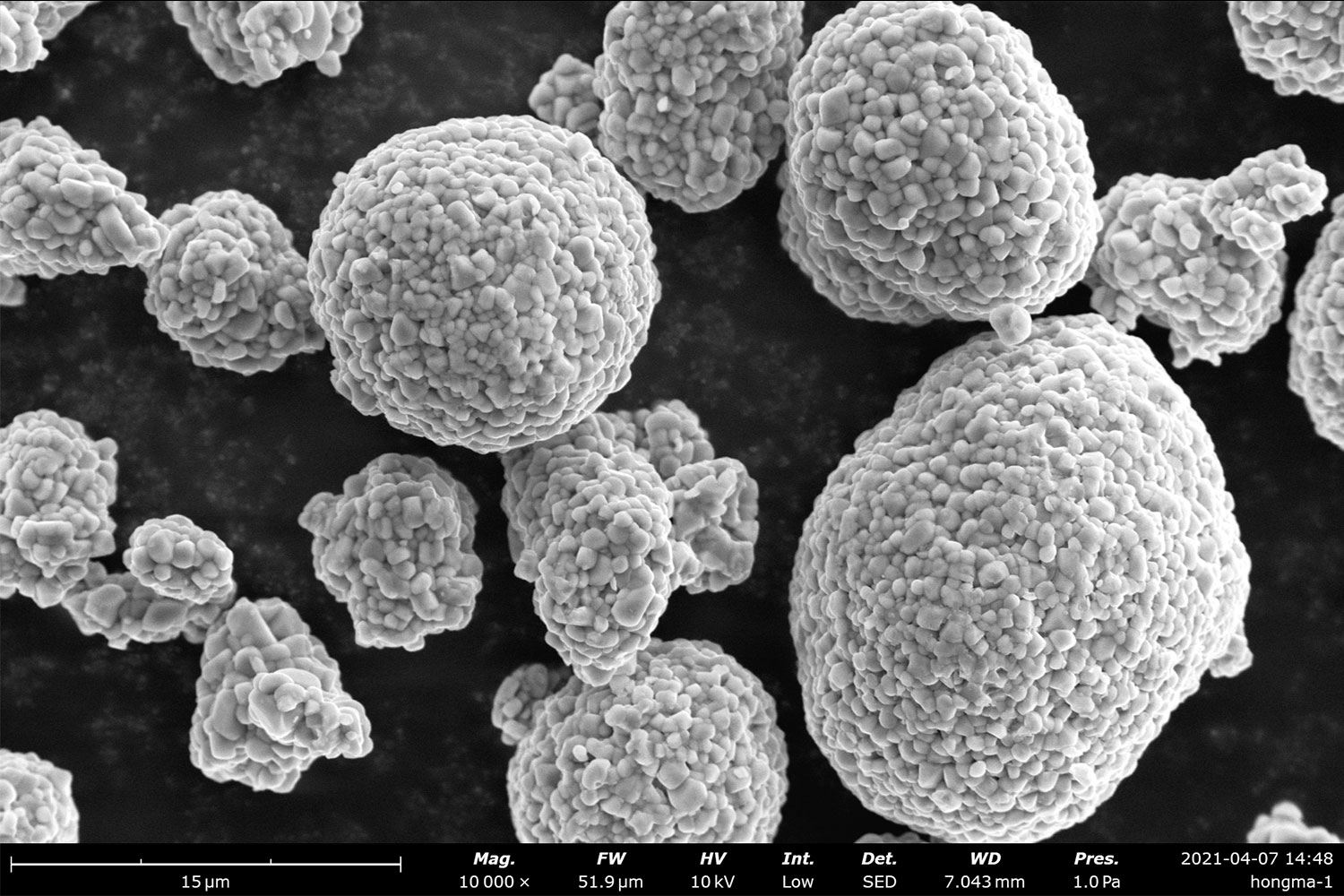
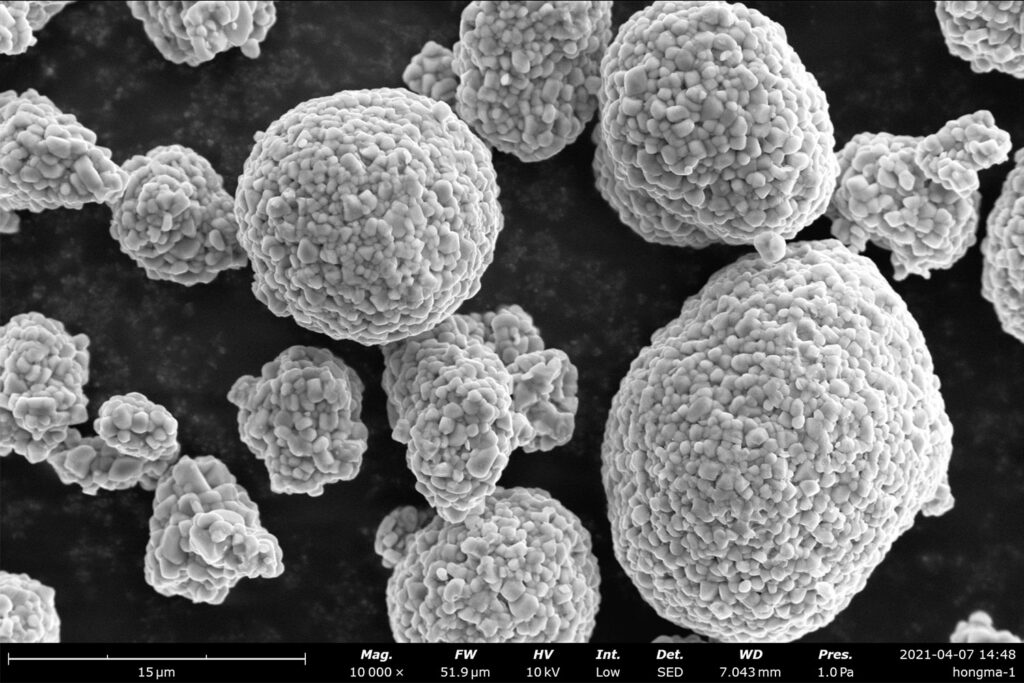
The functional properties of a battery, not limited to just its performance, are inherently dependent upon the microstructure and surface morphology of electrodes and separators. Scanning electron microscopy is a widely used tool in battery research to study the properties of components, as well as to investigate the interface between the electrode and electrolyte.
SEM is specifically applied to battery research in many overlapping ways.
Microstructural analysis
Feature sizing and structural defects
An important part of ensuring high electrode quality is developing an understanding of the particle size, shape, distribution, and structural integrity of battery electrodes. The electronic and ionic transport properties are influenced by the microstructures of each component, so this research naturally suits SEM due to the capability of probing at a higher resolution than any optical microscope.
Cathodes are commonly manufactured with NCM (lithium, nickel, cobalt, and manganese) particles. If the particle size is too large, transport is hindered by a higher diffusion length, while size distribution can influence the packing density of particles and uniformity of transport. In cases where the structural features of electrodes are of the highest interest, SEM excels as a tool for identifying crystalline defects, which in turn makes it an asset for researchers in avoiding structural cracks, pores, dendrites, and overall risk of failure.
Porous membranes referred to as separators reside in between the anode and cathode of batteries. The role of these materials is to prevent electrical shorting while encouraging efficient ion transport through the electrolyte. As such, the pore size and distribution are essential to study when examining the properties of battery separators. Taking measurements of these features is often quick and easy using SEM, and large amounts of data can be acquired with automated image analysis software programs that identify parameters like porosity and fiber thickness.

Surface and interface analysis
Surface roughness, topography, pore size, and distribution
Since each component of a battery is in direct contact with another, the surface roughness of each material is an important characteristic to monitor. The degree of roughness influences the surface area available for ion transport, ultimately swaying the electrochemical performance of the battery. SEMs provide clear insight into morphological parameters through high-resolution images, allowing direct visualization of surface features.

The interface between the electrode and electrolyte is easily probed with SEM and remains one of the most critical regions in a battery to study because of the formation of solid electrolyte interphase (SEI) layers. SEI layers are comprised of various inorganic (lithium and carbon, fluorine, oxygen, and hydrogen) and organic (carbonates) components, formed from electrochemical redox reactions between lithium ions and the electrolyte. As the layer forms, lithium ions are consumed, leaving the electrodes with a degraded charge cycle efficiency. However, SEI layers serve as conductors for ions and insulators for electrons – at least ideally. Therefore, investigating SEI layers during charge cycles is an important process to develop more efficient electrolyte-electrode systems, and is a process aided greatly by SEM.

How can SEMs accommodate air-sensitive samples?
Often in energy storage research, air-sensitive materials and volatile samples are susceptible to severe degradation caused by lithium oxidation. How useful would SEM be if a sample is compromised before the imaging process even begins? One solution is to perform the analysis within the glovebox itself, keeping the specimen surrounded by an inert gas until results are acquired.
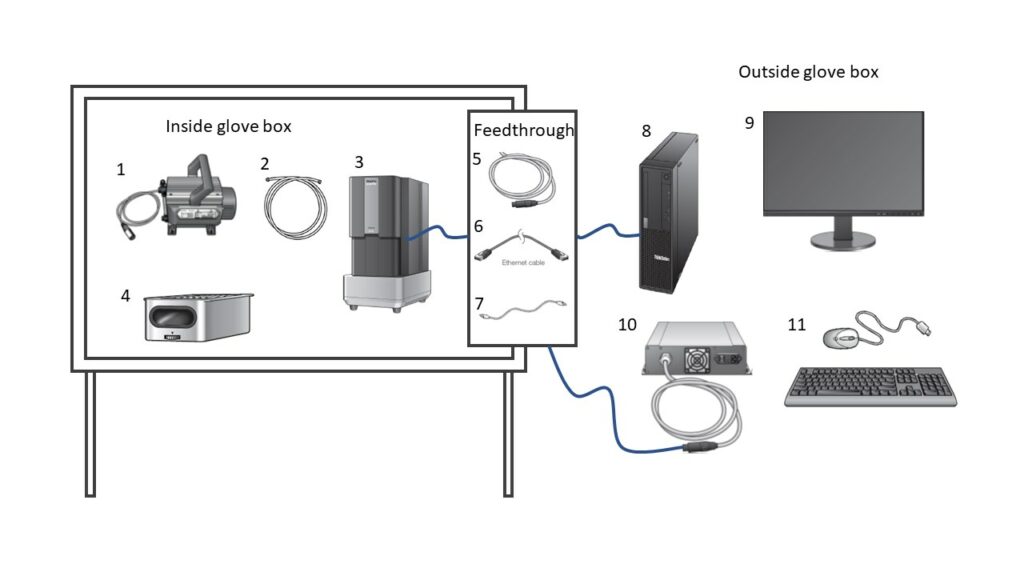
The Phenom XL argon-compatible desktop SEM is a unique system that is designed to operate within a glovebox. The compact design of a desktop system is crucial for fitting within the glovebox chamber, while strategic modifications to the components enable safe operation in the argon environment. The inert gas retains a level of protected atmosphere over samples to ensure quality control of samples during both preparation and analysis phases in the same location. The fundamental setup is highlighted in the image below with emphasis on what remains inside the glovebox compared to the remaining exterior points.
Argon-compatible glovebox SEM – Phenom XL